Figure 1.
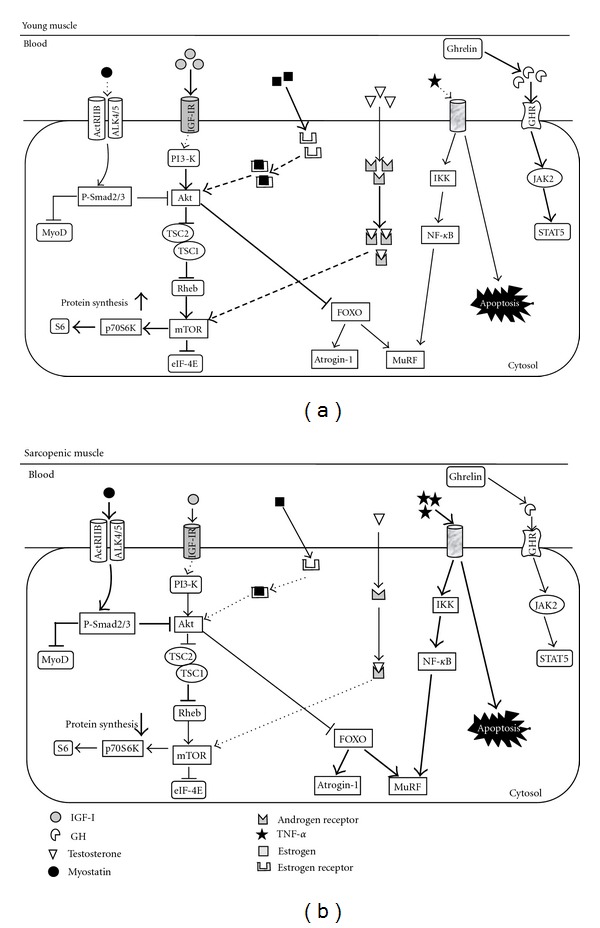
(a) In young muscle, abundant serum IGF-I can stimulate protein synthesis by activating Akt/mTOR/p70S6K pathway. Akt blocks the nuclear translocation of FOXO to inhibit the expression of Atrogin-1 and MuRF and the consequent protein degradation. Abundant serum GH, which is induced by ghrelin, activates JAK2-STAT5 signaling to promote muscle-specific gene transcription necessary to hypertrophy. In young muscle, testosterone and estrogen bind these intramuscular receptors (androgen receptor and estrogen receptor (α and β)), and activate mTOR and Akt, respectively. Lower serum amount of myostatin and TNF-α failed to activate signaling candidates (Smad 2/3, NF-κB, etc.) enhancing protein degradation. (b) In sarcopenic muscle, myostatin signals through the activin receptor IIB (ActRIIB), ALK4/5 heterodimer seems to activate Smad2/3 and blocking of MyoD transactivation in an autoregulatory feedback loop. Abundant activated Smad2/3 inhibit protein synthesis probably due to blocking the functional role of Akt. The increased blood TNF-α elevates the protein degradation through IKK/NF-κB signaling and enhance an apoptosis. Lower serum amount of IGF-I, GH, and anabolic hormones (testosterone and estrogen) failed to activate signaling candidates (Akt, mTOR, STAT5, etc.) enhancing protein synthesis. The impaired regulation of FOXO by Akt results in abundant expression of Atrogin-1 and MuRF and the consequent protein degradation in sarcopenic muscle.
