Abstract
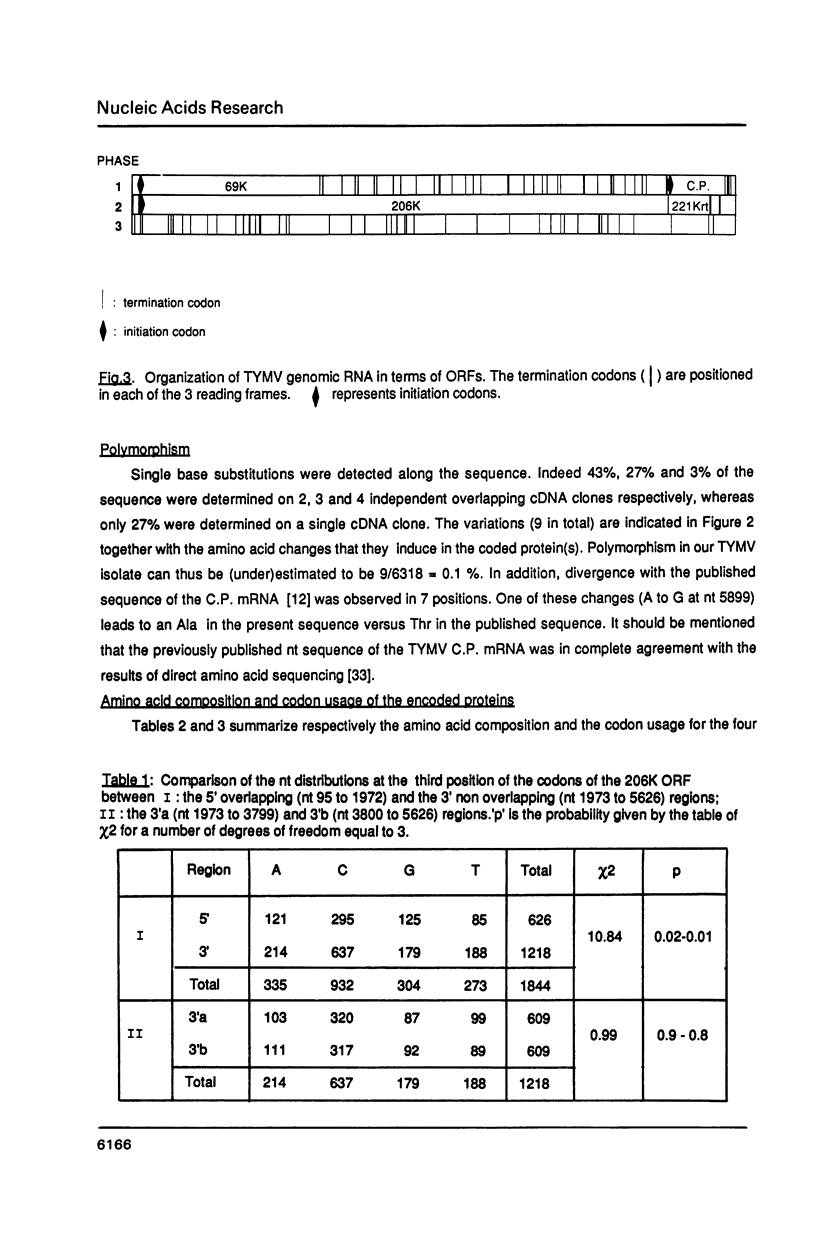
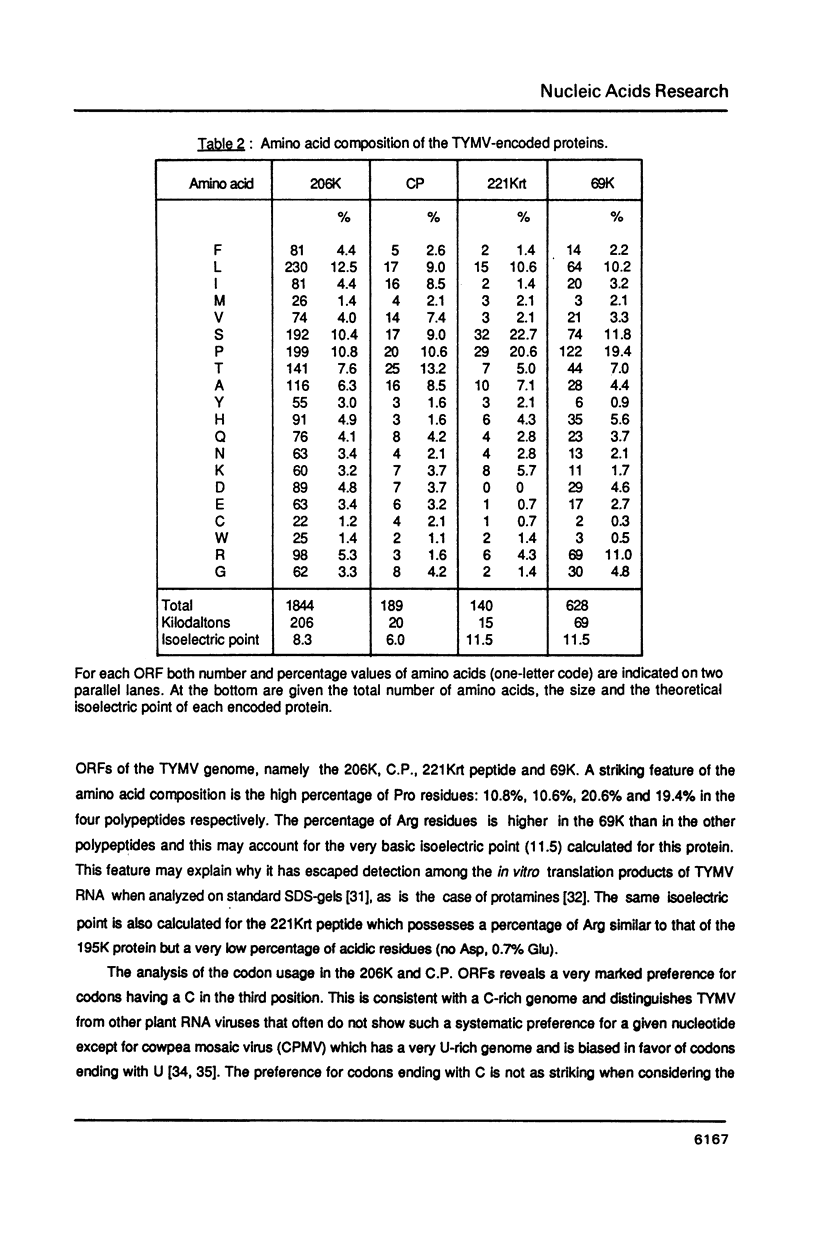
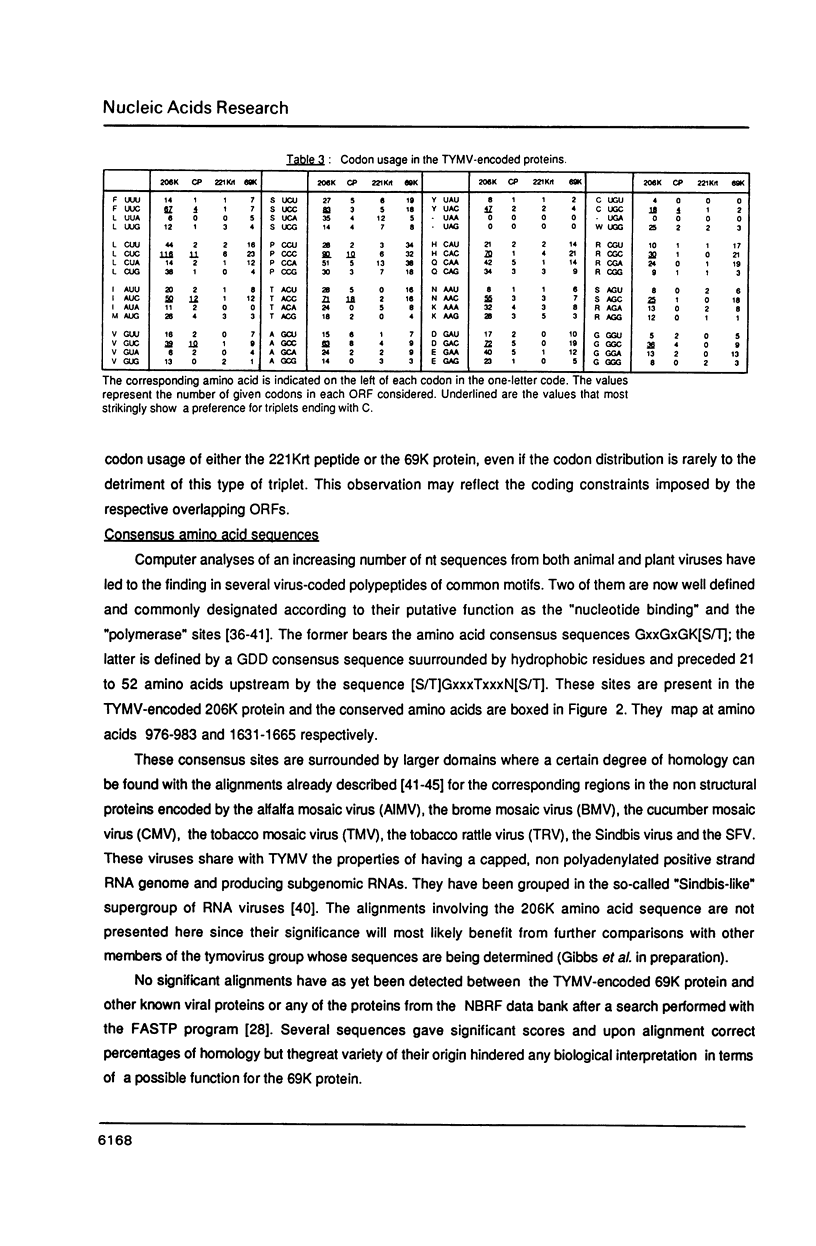
The complete nucleotide sequence of turnip yellow mosaic virus (TYMV) genomic RNA has been determined on a set of overlapping cDNA clones using a sequential sequencing strategy. The RNA is 6318 nucleotides long, excluding the cap structure. The genome organization deduced from the sequence confirms previous results of in vitro translation. A novel open reading frame (ORF) putatively encoding a Pro-rich and very basic 69K (K = kilodalton) protein is detected at the 5' end of the genome. It is initiated at the first AUG codon on the RNA and overlaps the major ORF that encodes the non structural 206K (previously referred to as 195K) protein of TYMV; its function is unknown. Several amino acid consensus sequences already described among plant and animal viruses are also found in the TYMV-encoded polypeptides. A comparison with other viruses whose RNA sequence is known leads to the conclusion that TYMV belongs to the "Sindbis-like" supergroup of viruses and could be related to Semliki forest virus.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlquist P., Strauss E. G., Rice C. M., Strauss J. H., Haseloff J., Zimmern D. Sindbis virus proteins nsP1 and nsP2 contain homology to nonstructural proteins from several RNA plant viruses. J Virol. 1985 Feb;53(2):536–542. doi: 10.1128/jvi.53.2.536-542.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argos P., Kamer G., Nicklin M. J., Wimmer E. Similarity in gene organization and homology between proteins of animal picornaviruses and a plant comovirus suggest common ancestry of these virus families. Nucleic Acids Res. 1984 Sep 25;12(18):7251–7267. doi: 10.1093/nar/12.18.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes W. M. Plasmid detection and sizing in single colony lysates. Science. 1977 Jan 28;195(4276):393–394. doi: 10.1126/science.318764. [DOI] [PubMed] [Google Scholar]
- Benicourt C., Haenni A. L. Differential translation of turnip yellow mosaic virus mRNAs in vitro. Biochem Biophys Res Commun. 1978 Oct 30;84(4):831–839. doi: 10.1016/0006-291x(78)91659-5. [DOI] [PubMed] [Google Scholar]
- Boccara M., Hamilton W. D., Baulcombe D. C. The organisation and interviral homologies of genes at the 3' end of tobacco rattle virus RNA1. EMBO J. 1986 Feb;5(2):223–229. doi: 10.1002/j.1460-2075.1986.tb04202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briand J. P., Jonard G., Guilley H., Richards K., Hirth L. Nucleotide sequence (n=159) of the amino-acid-accepting 3'-OH extremity of turnip-yellow-mosaic-virus RNA and the last portion of its coat-protein cistron. Eur J Biochem. 1977 Feb;72(3):453–463. doi: 10.1111/j.1432-1033.1977.tb11269.x. [DOI] [PubMed] [Google Scholar]
- Briand J. P., Keith G., Guilley H. Nucleotide sequence at the 5' extremity of turnip yellow mosaic virus genome RNA. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3168–3172. doi: 10.1073/pnas.75.7.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bénicourt C., Péré J. P., Haenni A. L. Translation of TYMV RNA into high molecular weight proteins. FEBS Lett. 1978 Feb 15;86(2):268–272. doi: 10.1016/0014-5793(78)80577-8. [DOI] [PubMed] [Google Scholar]
- Craigen W. J., Caskey C. T. Translational frameshifting: where will it stop? Cell. 1987 Jul 3;50(1):1–2. doi: 10.1016/0092-8674(87)90652-0. [DOI] [PubMed] [Google Scholar]
- Domingo E., Martínez-Salas E., Sobrino F., de la Torre J. C., Portela A., Ortín J., López-Galindez C., Pérez-Breña P., Villanueva N., Nájera R. The quasispecies (extremely heterogeneous) nature of viral RNA genome populations: biological relevance--a review. Gene. 1985;40(1):1–8. doi: 10.1016/0378-1119(85)90017-4. [DOI] [PubMed] [Google Scholar]
- Elsevier S. M. Messenger RNA encoding basic chromosomal proteins of mouse testis. Dev Biol. 1982 Mar;90(1):1–12. doi: 10.1016/0012-1606(82)90205-6. [DOI] [PubMed] [Google Scholar]
- Goad W. B., Kanehisa M. I. Pattern recognition in nucleic acid sequences. I. A general method for finding local homologies and symmetries. Nucleic Acids Res. 1982 Jan 11;10(1):247–263. doi: 10.1093/nar/10.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbach R. Genome similarities between plant and animal RNA viruses. Microbiol Sci. 1987 Jul;4(7):197–202. [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Guilley H., Briand J. P. Nucleotide sequence of turnip yellow mosaic virus coat protein mRNA. Cell. 1978 Sep;15(1):113–122. doi: 10.1016/0092-8674(78)90087-9. [DOI] [PubMed] [Google Scholar]
- Haseloff J., Goelet P., Zimmern D., Ahlquist P., Dasgupta R., Kaesberg P. Striking similarities in amino acid sequence among nonstructural proteins encoded by RNA viruses that have dissimilar genomic organization. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4358–4362. doi: 10.1073/pnas.81.14.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidecker G., Messing J. Sequence analysis of zein cDNAs obtained by an efficient mRNA cloning method. Nucleic Acids Res. 1983 Jul 25;11(14):4891–4906. doi: 10.1093/nar/11.14.4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Hong G. F. A systemic DNA sequencing strategy. J Mol Biol. 1982 Jul 5;158(3):539–549. doi: 10.1016/0022-2836(82)90213-3. [DOI] [PubMed] [Google Scholar]
- Kamer G., Argos P. Primary structural comparison of RNA-dependent polymerases from plant, animal and bacterial viruses. Nucleic Acids Res. 1984 Sep 25;12(18):7269–7282. doi: 10.1093/nar/12.18.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C., Fritsch C., Briand J. P., Richards K. E., Jonard G., Hirth L. Physical and functional heterogeneity in TYMV RNA: evidence for the existence of an independent messenger coding for coat protein. Nucleic Acids Res. 1976 Nov;3(11):3043–3061. doi: 10.1093/nar/3.11.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leberman R. The isolation of plant viruses by means of "simple" coacervates. Virology. 1966 Nov;30(3):341–347. doi: 10.1016/0042-6822(66)90112-7. [DOI] [PubMed] [Google Scholar]
- Lin H. C., Lei S. P., Wilcox G. An improved DNA sequencing strategy. Anal Biochem. 1985 May 15;147(1):114–119. doi: 10.1016/0003-2697(85)90016-8. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Lomonossoff G. P., Shanks M. The nucleotide sequence of cowpea mosaic virus B RNA. EMBO J. 1983;2(12):2253–2258. doi: 10.1002/j.1460-2075.1983.tb01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lütcke H. A., Chow K. C., Mickel F. S., Moss K. A., Kern H. F., Scheele G. A. Selection of AUG initiation codons differs in plants and animals. EMBO J. 1987 Jan;6(1):43–48. doi: 10.1002/j.1460-2075.1987.tb04716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malthiery B., Bellon B., Giorgi D., Jacq B. Apple II PASCAL programs for molecular biologists. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):569–579. doi: 10.1093/nar/12.1part2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshi T., Ishikawa M., Motoyoshi F., Semba K., Okada Y. In vitro transcription of infectious RNAs from full-length cDNAs of tobacco mosaic virus. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5043–5047. doi: 10.1073/pnas.83.14.5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morch M. D., Benicourt C. Polyamines stimulate suppression of amber termination codons in vitro by normal tRNAs. Eur J Biochem. 1980 Apr;105(3):445–451. doi: 10.1111/j.1432-1033.1980.tb04519.x. [DOI] [PubMed] [Google Scholar]
- Morch M. D., Benicourt C. Post-Translational Proteolytic Cleavage of In Vitro-Synthesized Turnip Yellow Mosaic Virus RNA-Coded High-Molecular-Weight Proteins. J Virol. 1980 Apr;34(1):85–94. doi: 10.1128/jvi.34.1.85-94.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morch M. D., Zagórski W., Haenni A. L. Proteolytic maturation of the turnip-yellow-mosaic-virus polyprotein coded in vitro occurs by internal catalysis. Eur J Biochem. 1982 Oct;127(2):259–265. doi: 10.1111/j.1432-1033.1982.tb06864.x. [DOI] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Paul H. L., Gibbs A., Wittman-Liebold B. The relationship of certain tymoviruses assessed from the amino acid composition of their coat proteins. Intervirology. 1980;13(2):99–109. doi: 10.1159/000149114. [DOI] [PubMed] [Google Scholar]
- Peter R., Stehelin D., Reinbolt J., Collot D., Duranton H. Primary structure of turnip yellow mosaic virus coat protein. Virology. 1972 Aug;49(2):615–617. doi: 10.1016/0042-6822(72)90516-8. [DOI] [PubMed] [Google Scholar]
- Pleij C. W., Neeleman A., van Vloten-Doting L., Bosch L. Translation of turnip yellow mosaic virus RNA in vitro: a closed and an open coat protein cistron. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4437–4441. doi: 10.1073/pnas.73.12.4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poncz M., Solowiejczyk D., Ballantine M., Schwartz E., Surrey S. "Nonrandom" DNA sequence analysis in bacteriophage M13 by the dideoxy chain-termination method. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4298–4302. doi: 10.1073/pnas.79.14.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter A., Carey N., Fellner P. Presence of a large poly(rC) tract within the RNA of encephalomyocarditis virus. Nature. 1974 Apr 19;248(5450):675–678. doi: 10.1038/248675a0. [DOI] [PubMed] [Google Scholar]
- Rezaian M. A., Williams R. H., Gordon K. H., Gould A. R., Symons R. H. Nucleotide sequence of cucumber-mosaic-virus RNA 2 reveals a translation product significantly homologous to corresponding proteins of other viruses. Eur J Biochem. 1984 Sep 3;143(2):277–284. doi: 10.1111/j.1432-1033.1984.tb08370.x. [DOI] [PubMed] [Google Scholar]
- Ricard B., Barreau C., Renaudin H., Mouches C., Bové J. M. Messenger properties of TYMV-RNA. Virology. 1977 Jun 1;79(1):231–235. doi: 10.1016/0042-6822(77)90347-6. [DOI] [PubMed] [Google Scholar]
- Strauss E. C., Kobori J. A., Siu G., Hood L. E. Specific-primer-directed DNA sequencing. Anal Biochem. 1986 Apr;154(1):353–360. doi: 10.1016/0003-2697(86)90536-1. [DOI] [PubMed] [Google Scholar]
- Strauss E. G., Rice C. M., Strauss J. H. Complete nucleotide sequence of the genomic RNA of Sindbis virus. Virology. 1984 Feb;133(1):92–110. doi: 10.1016/0042-6822(84)90428-8. [DOI] [PubMed] [Google Scholar]
- Takkinen K. Complete nucleotide sequence of the nonstructural protein genes of Semliki Forest virus. Nucleic Acids Res. 1986 Jul 25;14(14):5667–5682. doi: 10.1093/nar/14.14.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yanofsky C. Attenuation in the control of expression of bacterial operons. Nature. 1981 Feb 26;289(5800):751–758. doi: 10.1038/289751a0. [DOI] [PubMed] [Google Scholar]
- Zagorski W., Morch M. D., Haenni A. L. Comparison of three different cell-free systems for turnip yellow mosaic virus RNA translation. Biochimie. 1983 Feb;65(2):127–133. doi: 10.1016/s0300-9084(83)80183-7. [DOI] [PubMed] [Google Scholar]
- van Wezenbeek P., Verver J., Harmsen J., Vos P., van Kammen A. Primary structure and gene organization of the middle-component RNA of cowpea mosaic virus. EMBO J. 1983;2(6):941–946. doi: 10.1002/j.1460-2075.1983.tb01525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


