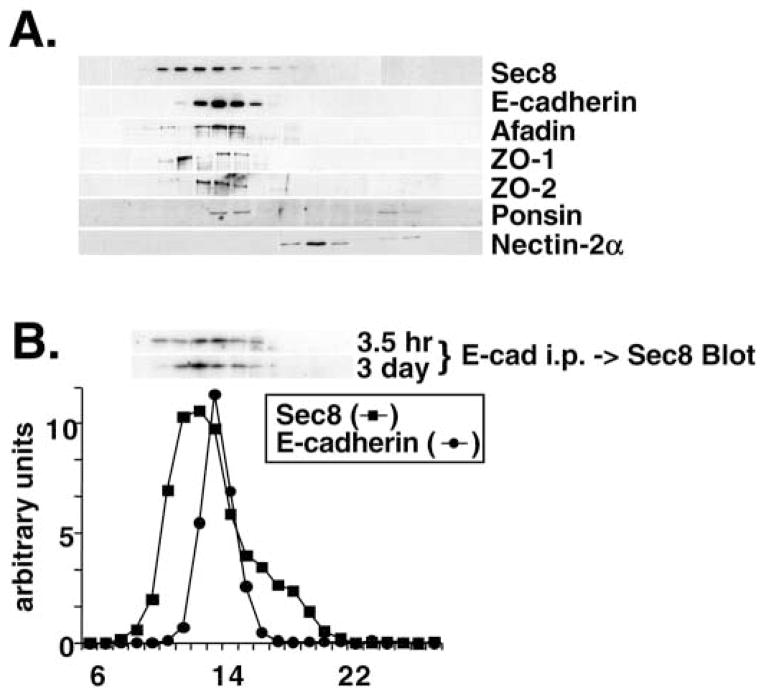
Fig. 5.
Fractionation of junctional proteins associated with Sec6/8 complex. (A) Detergent extracts of polarized MDCK cells were fractionated by Superose 6 FPLC as described in Materials and Methods. Fractions 6–28 were divided into equal aliquots, separated by SDS-PAGE, and transferred to Immobilon P membranes. Membranes were probed with antibodies to Sec8, E-cadherin, afadin, ZO-1, ZO-2, ponsin or nectin-2α. Protein levels were quantified using a Molecular Dynamics Phosphorimager. The elution profiles of Sec6 and E-cadherin are shown in B. The elution peaks of globular protein standards with known relative molecular masses were also determined: thyroglobulin, Mr=669,000 (fraction 16); apoferritin, Mr=443,000 (fraction 19); catalase, Mr=232,000 (fraction 22); bovine serum albumin, Mr=66,000 (fraction 24). (B) Coimmunoprecipitation of Sec8 with E-cadherin adhesion complex. MDCK cells were extracted either 3.5 hours or 3 days after induction of calcium-dependent cell-cell adhesion and extracts were fractionated by Superose 6 FPLC. Each fraction (10–19) was subjected to immunoprecipitation with anti-E-cadherin E2 antiserum. Immunoprecipitated material was eluted in SDS-PAGE sample buffer and the presence of Sec8 in each fraction was assayed by SDS-PAGE followed by immunoblotting. Protein levels were quantified using a Molecular Dynamics Phosphorimager.