Abstract
The formation of functional synapses on artificial substrates is a very important step in the development of engineered in vitro neural networks. Spherical supported bilayer lipid membranes (SS-BLMs) are used here as a novel substrate to demonstrate presynaptic vesicle accumulation at an in vitro synaptic junction. Confocal fluorescence microscopy, cryo-transmission electron microscopy (cryo-TEM), and fluorescence recovery after photobleaching (FRAP) experiments have been used to characterize the SS-BLMs. Conventional immunocytochemistry combined with confocal fluorescence microscopy was used to observe the formation of presynaptic vesicles at the neuron−SS-BLM contacts. These results indicate that lipid phases may play a role in the observed phenomenon, in addition to the chemical and electrostatic interactions between the neurons and SS-BLMs. The biocompatibility of lipid bilayers along with their membrane tunability makes the suggested approach a useful “toolkit” for many neuroengineering applications including artificial synapse formation and synaptogenesis in vivo.
Keywords: Lipid membranes, supported bilayers, neurons, synapse, neurodegenerative diseases, regenerative medicine
The complexity of the central nervous system (CNS) makes it difficult to repair damaged neuronal pathways using conventional tissue engineering approaches (1). This challenge has stimulated a number of new approaches (2). Critical components of any succcessful engineered neural network will include controlled neurite outgrowth guidance, the development of interneuronal contacts on specific artificial substrates, and the formation of functional synapses at the synaptic junctions. We have recently shown that presynaptic vesicle assembly at an in vitro synaptic junction does not require postsynaptic factors (3). Two decades ago Burry et al. showed that presynaptic-like endings can form when axons are directed onto polylysine (PL)-coated latex beads (4). Our interest in forming artificial synapses in vitro prompted a detailed study of this configuration and variants thereof. Functional synapses are indeed formed following adhesion of axon terminals of hippocampal neurons when they are cocultured with poly-d-lysine (PDL)-coated latex beads to axonal shafts (3). This work allows us to explore the range of factors that are important in synapse formation in vitro. Synthetic lipid bilayer membranes are interesting candidates in this regard due to their close structural similarity with pre- and postsynaptic membranes (5).
This paper describes our observation that phospholipids in the form of solid supported bilayer lipid membranes (SS-BLMs) formed on silica beads (6,7), when rationally designed, can be used as an artificial substrate for in vitro synapse formation. This observation not only is interesting in developing potential substrates for in vitro synapse formation but also will provide us new insights into the role that physical and mechanical properties of cell membranes may play at synapses. SS-BLMs derived from synthetic lipid bilayers offer many opportunities to tailor the physical, chemical, and functional properties of an ideal artificial substrate (8,9). When living cells interact with these SS-BLM-derived substrates, the induced cell responses will be dictated by the chemical and physical properties of the lipid bilayer membrane used as well as the particular cell type (10). Although Groves et al. have used SS-BLMs as scaffolds for incorporating neurologically active neuroligin-1 molecules to target β-neurexin receptors in ligand−receptor interaction studies (9), in particular for immunosynapse formation studies (11), their role in tuning neuron−scaffold interactions has not yet been reported.
Figure 1a shows a sketch of a typical SS-BLM, formed on silica beads, used in the experiments illustrated in this communication. We have used a previously established procedure (6) for the preparation of these SS-BLMs. Figure 1b is a representative confocal fluorescence image showing an SS-BLM-coated silica bead (the term SS-BLM/bead is used hereafter, unless stated otherwise) where the fluorescence is derived from tetramethyl rhodamine isothiocyanate (TRITC)-labeled phosphatidylethanolamine incorporated in the bilayer. The uniform fluorescence observed indicates the formation of SS-BLMs on the beads (see also Figure SI.1, Supporting Information for an additional confocal image of a population of SS-BLM/beads). Figure 1d is a representative cryo-TEM image of a mixed SS-BLM/bead formed on 500 nm silica beads. A small area is highlighted in Figure 1f for better visualization, where the presence of a bilayer is clearly visible. For comparison, an uncoated silica bead is shown in Figure 1c,e (see Figure SI.2, Supporting Information for a clearer visualization of the bilayer that spans the entire bead surface). Fluorescence recovery after photobleaching (FRAP) (9) imaging was performed to further confirm the quality of the membranes on SS-BLMs used in this study (time series included as an AVI file). Thorough characterization of these SS-BLMs (various bead sizes; different lipid compositions, etc.) using different microscopy/spectroscopy techniques has already been documented in our prior publication (6).
Figure 1.
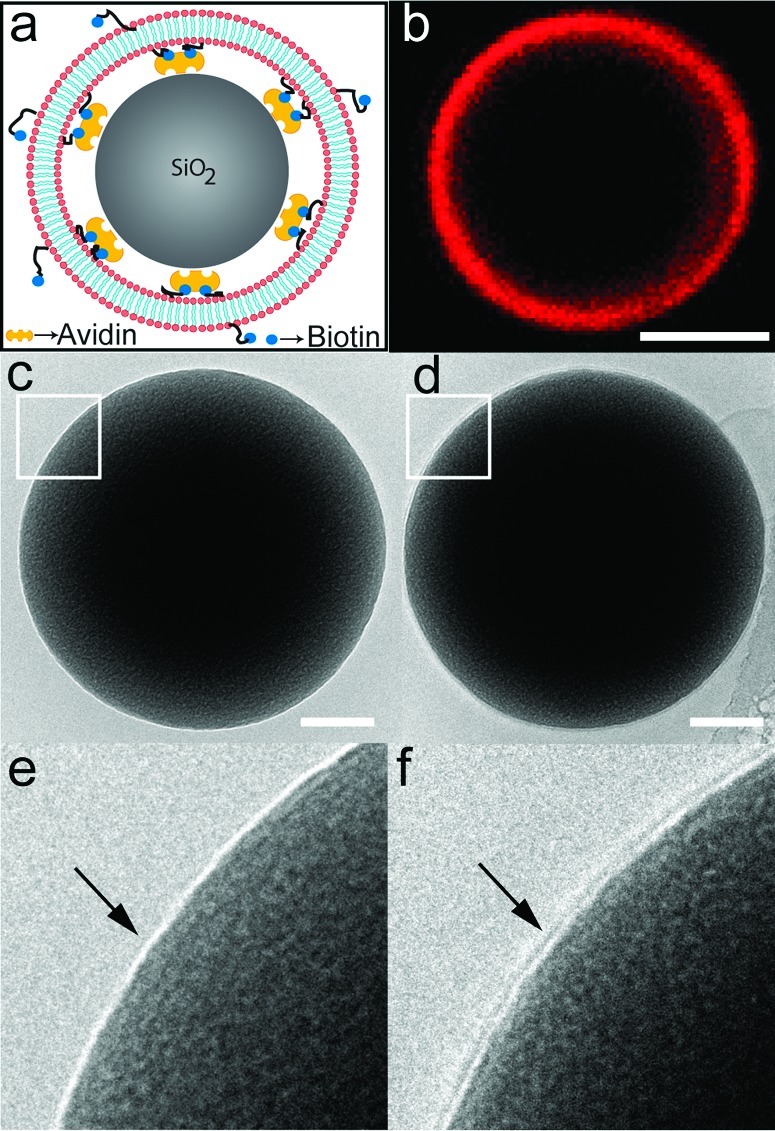
Preparation and characterization of SS-BLM/beads composed of DOPC/DOTAP/DPPE (25:25:50) in the bilayer. (a) Sketch (not to scale) depicting a cross-sectional view of the suggested molecular orientation of SS-BLMs on silica beads. (b) Confocal cross-sectional image of a 5 μm SS-BLM/bead (see also figure SI.1, Supporting Information). The fluorescence is derived from 0.1 mol % N-(6-tetramethylrhodaminethiocarbamoyl)-1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine, triethylammonium salt (TRITC−DHPE) used in the bilayer (scale bar is 2 μm). Cryo-TEM images of free-standing, vitrified samples of 500 nm (c) uncoated and (d) SS-BLM-coated silica beads (scale bars are 100 nm). Images e and f show close-up views of the selected areas (white squares) in images c and d, respectively (see also Figure SI.2, Supporting Information).
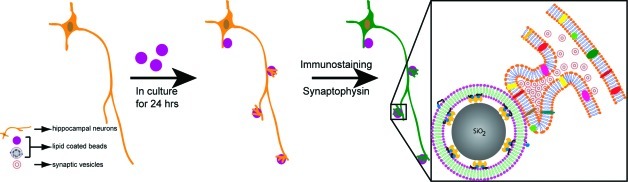
The use of these SS-BLM/beads to induce in vitro synapses upon contact with live axons was evaluated with a number of different lipid mixtures. In a typical procedure, SS-BLM/beads, prepared under sterile conditions, were cocultured with hippocampal neurons (7+ days in vitro (DIV)) for 24 h at 37 °C, 5% CO2. The cocultured cells were then fixed and fluorescently stained primarily for synaptophysin (an integral synaptic vesicle protein) using immunocytochemistry and observed using confocal fluorescence microscopy. The overall experimental procedure in the neuron−SS-BLM/bead coculture is depicted in Scheme 1.
Scheme 1. Scheme (Not to Scale) Illustrating the General Experimental Protocol Used in This Work To Observe the “Synaptic Boutons” Formed on SS-BLM/Beads.
Fluorescently labeled secondary antibodies that bind specifically to synaptophysin primary antibodies via an immunostaining protocol were used primarily to follow the formation of the “synaptic boutons”. This particular sketch depicts a “positive staining” for synaptophysin (seen in synaptic vesicle membranes). In the case of “negative staining”, the experimental steps remain the same except that synaptic vesicle (small red circles) accumulation will be absent, and thus no fluorescence will be observed around the beads.
An SS-BLM composed of a binary mixture of a zwitterionic phospholipid and a cationic lipid (1,2-dioleoyl-sn-glycero-3-phosphatidylcholine (DOPC)/1,2-dioleoyl-3-trimethylammonium-propane (DOTAP); 75:25, all lipid ratios are shown in molar ratios unless stated otherwise) promotes the adhesion of beads to the hippocampal neurons (Figure 2b). DOTAP is a cationic lipid that has been used extensively in DNA transfection experiments. It is widely believed that the positive charge on DOTAP plays a major role in establishing electrostatic attraction between biological cells and the transfection formulations before other mechanisms become operative (12). In a similar manner, a positive charge is thus required for a strong bead−axon adhesive interaction. As seen in Figure 2b, almost all of these cationic beads become attached to the axons. However, the formally neutral (zwitterionic) DOPC (100%) SS-BLM/beads (Figure 2a) are almost completely removed by washing steps indicating that the adhesion established between the neutral SS-BLM/beads and the neurons is weak or negligible (10b). In both cases, however, no significant neurite responses are observed (Figures 2d,f). As described later, only when adhesion occurs do other functionalities associated with the lipid bilayer induce any neural responses with an axonal membrane.
Figure 2.
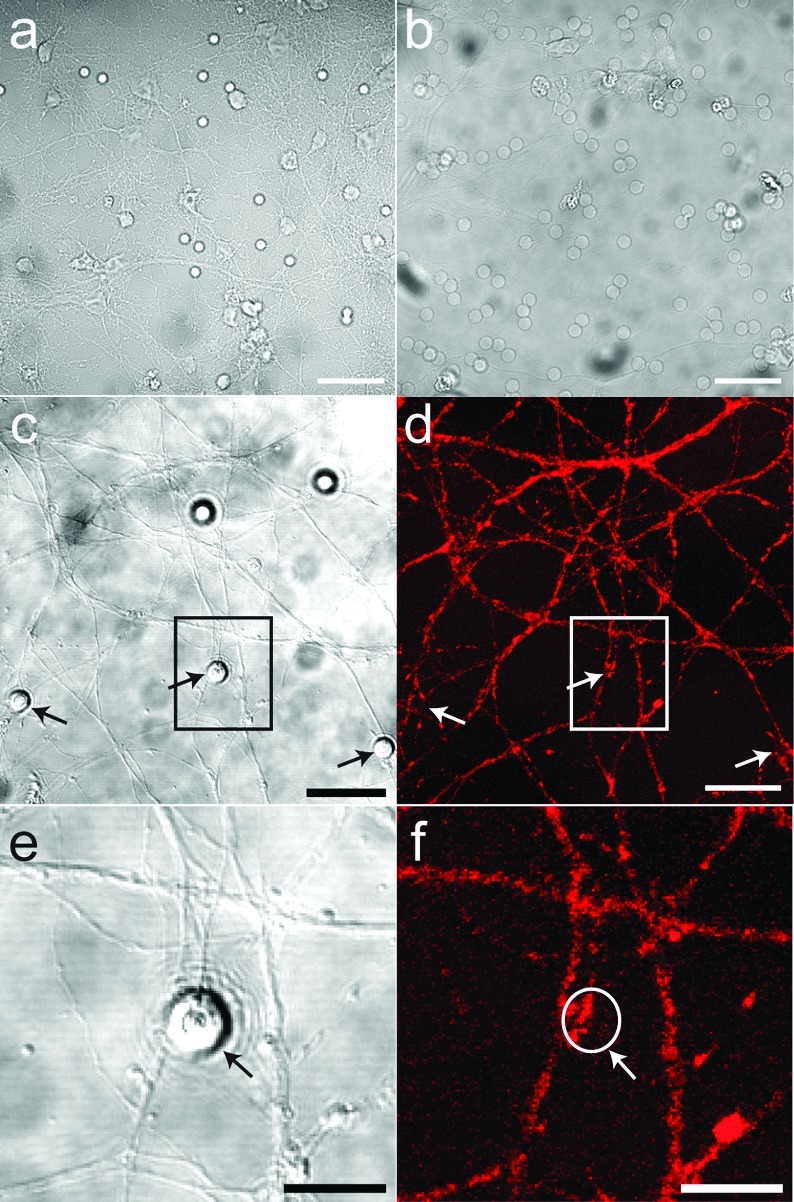
Representative diffusion interference contrast (DIC) (a−c,e) and corresponding confocal fluorescence (d,f) images of hippocampal neurons cocultured with SS-BLM/beads: (a) DIV16 cells after 24 h incubation with DOPC SS-BLM/beads; (b) DIV18 cells after 24 h incubation with DOPC/DOTAP (75:25) SS-BLM/beads—compared with image a, image b shows clear adhesion of beads to the neurites in culture; (c,d) DIV14 cells after 24 h incubation with DOPC/DOTAP (75:25) SS-BLM/beads. As is evidenced from the image, there is no presynaptic response at the bead−axon contact sites. The fluorescent boutons seen along the axons (d) are presynaptic vesicles accumulated at synaptic junctions formed between the axons and PDL used for cell adhesion on the glass substrate. Images e and f show magnified views of areas marked in images c and d. The white circle in image f shows the exact location of the beads in culture for better visualization. Neurons immunostained after incubation with DOPC SS-BLMs yielded similar results. The cells were fixed and incubated with antisynaptophysin primary antibodies (rabbit polyclonal), which were subsequently stained using Alexa-543 tagged antirabbit secondary antibodies. Scale bars are 25 μm (a−d) and 10 μm (e, f).
Polylysine adsorbed onto latex beads also exerts presynaptic vesicle recruitment at the points of contact (3,4). In addition to its known capability to effect strong adhesion with cell membranes, PDL-induced neurite responses are likely through some form of chemoselective process via the primary amine moieties. We thus introduced primary amines into the SS-BLM via a phosphatidylethanolamine lipid (PE lipid). Little effect was noted when a ternary lipid mixture (DOPC/DOTAP/(1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine (DPPE); 50:25:25) was used in neuronal culture (data not shown) in place of the 75:25 DOPC/DOTAP SS-BLM (Figure 2d). However, when the DPPE concentration was increased to 50 mol % in the ternary lipid mixture (DOPC/DOTAP/DPPE; 25:25:50; Figures 1d,f and 3) the beads are effective in the recruitment of presynaptic vesicles at the bead−axon contacts. Most of the beads in the cell culture are positive for synaptophysin labeling (Figure 3), indicating that accumulation of presynaptic vesicles has occurred at the point of contact between the axons and the SS-BLM/beads.
Figure 3.
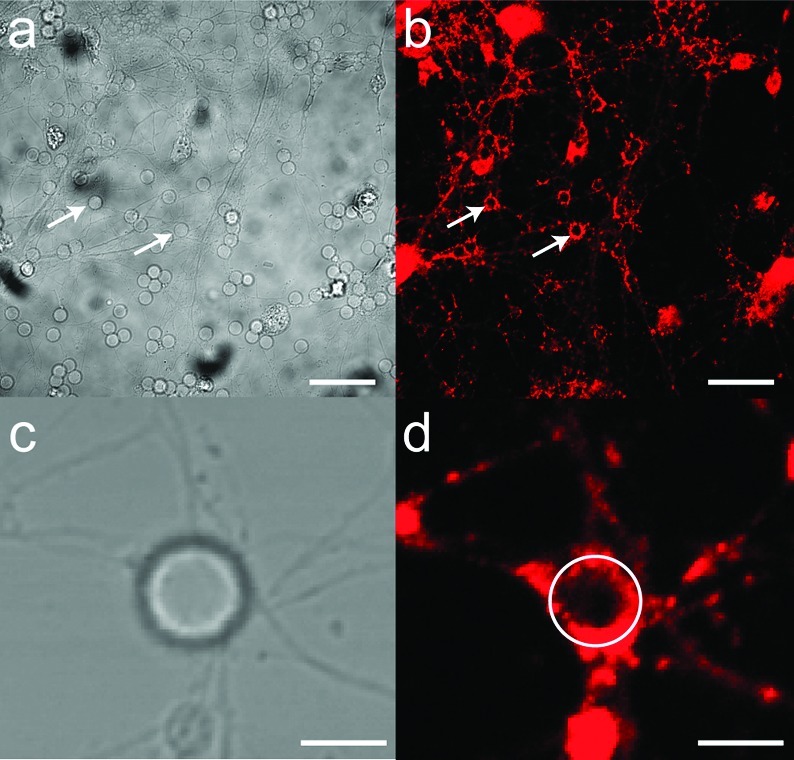
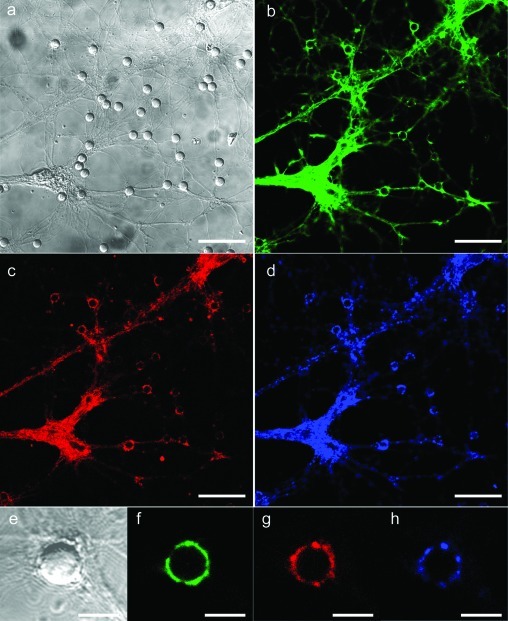
Representative confocal fluorescence (b, d) and the corresponding DIC (a, c) images of hippocampal neurons cocultured with SS-BLM/beads: (a−d) DIV18 cells after 24 h incubation with DOPC/DOTAP/DPPE (25:25:50) SS-BLM/beads are shown. Lower images (c, d) show a single bead in culture, and the white circle in image d shows the exact location of the bead in culture for better visualization. Image d in fact shows the extent of accumulation of presynapses occurring at the bead−axon contacts in comparison to those that occur in culture. The fluorescent boutons (on the glass substrate and on silica beads) are presynaptic vesicles expressing synaptophysin (b, d). The cells were fixed and incubated with antisynaptophysin primary antibodies (rabbit polyclonal), which were subsequently stained using Alexa-488 (b) and Alexa-543 (d) tagged antirabbit secondary antibodies. Scale bars are 25 μm (a, b) and 5 μm (c, d).
In order to track the presence of other important synaptic proteins at the bead−axon contacts, we performed a triple staining of our SS-BLM/bead−hippocampal neuron coculture. Figure 4 clearly shows that actin filaments (13) (panels b and f), which are important cytoskeletal components involved in many synaptic processes including signal transduction and synaptic vesicle trafficking, and bassoon (14) (panels d and h), an important scaffolding protein present at presynaptic active zones, are present at bead−axon contacts in addition to synaptophysin (Figures 3 and 4c,g). This was observed only in the case of SS-BLM/beads having a membrane composition of DOPC/DOTAP/DPPE (25:25:50). Control experiments performed using SS-BLM/beads having a membrane composition of DOPC/DOTAP (75:25) were negative for all three of these synaptic proteins (Figure SI.3, Supporting Information) when cocultured with hippocampal neurons. Immunostaining of PSD-95, a postsynaptic scaffolding protein, has been performed to verify whether postsynaptic factors were present (Figure SI.4, Supporting Information). The result implies, as in our prior work (3), that exclusive formation of postsynaptic terminals at the bead−axon contacts is absent. These observations are in good agreement with our earlier report that PDL-coated latex beads could trigger the formation of functional presynaptic endings at the bead−axon contacts (3). The presence of DPPE is clearly playing a role in the promotion of actin cytoskeletal networking at the bead−axon junctions and possibly in the subsequent recruitment of other synaptic proteins such as synaptophysin and bassoon. This is intriguing and is the subject of our ongoing study in the further understanding of the chemical/mechanical interplay of synaptic membranes at in vitro synaptic junctions.
Figure 4.
Representative confocal cross-sectional images (b−d and f−h) showing the presence of different synaptic proteins at the bead−axon contact points in a single culture. This experiment was performed using SS-BLM/beads containing of DOPC/DOTAP/DPPE, 25:25:50 (as per Figure 3), in the bilayer when cocultured with hippocampal neurons (DIV 14). The fluorescence clustering (boutons) is seen along the neurites along with an enhanced clustering on the beads that are in contact with the axons. The cells are fixed and incubated with (b) phalloidin−Alexa-488 (green channel) for actin filaments, (c) antisynaptophysin/α-rabbit−Alexa-543 (red channel) for synaptophysin, and (d) antibassoon/α-mouse−Cy-5 (blue channel) for bassoon. The DIC channel (a) shows the exact locations of SS-BLM/beads in the culture. Lower panels (e−f) show a single bead in culture. The images here were acquired at a slightly higher plane compared with the other figures for better visualization of fluorescence around the beads. Scale bars are 25 μm (a−d) and 5 μm (e−h).
The observation of presynaptic vesicle clustering at the bead site is very promising especially given the ongoing attempts to design more suitable and patterned substrates for in vitro neuroengineering applications. To this end, a better understanding of the underlying molecular mechanisms of synapse formation (14) is crucial, especially because recent observations suggest that even in the absence of postsynaptic factors, functional presynapses can be formed onto tailored substrates (3). SS-BLMs are clearly a viable substrate due to their structural similarity to cell membranes as well as numerous possibilities of membrane modification.
Lipid microdomains (rafts) (15a) in the cell plasma membrane are believed to play an important role in controlling many cellular events including cell signaling (15) and the guidance of neuronal growth cones (16). The DOPC/DOTAP/DPPE (25:25:50) SS-BLM/beads formed here are likely to exhibit lipid domain formation (5,17) given that the Tm of DOPC is −20 °C, whereas the Tm of DPPE is 63 °C. At the experimental temperature of 37 °C, lipid phase separation is thus likely to occur (17a). In order to observe whether a PE lipid whose Tm is closer to the matrix DOPC would produce similar results, we used 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) (Tm = −16 °C) in control experiments. It suggests that the effect exerted by DOPE on hippocampal neurons is very different from that exerted by DPPE. When DOPE is used, very few synaptic boutons (as evidenced by less clustering, Figure SI.5, Supporting Information) are observed. This demonstrates that in addition to the chemical specificity, lipid phase domains may play a very important role in the observed in vitro synapse formation. Further studies employing imaging techniques (17) that involve lipid domain-specific fluorescent markers are however necessary to establish whether lipid domains are present in the SS-BLM/beads and whether the presynaptic boutons colocalize with a specific lipid phase. Experiments in this regard are currently underway in our laboratory.
Important control experiments also confirm that (i) the possibility that membrane fusion events may play a role in the observed in vitro presynapse formation can be excluded (Figure SI.6, Supporting Information) and (ii) the bilayer membranes on SS-BLM/beads are stable after 24 h of bead−neuron coculture (Figure SI.7, Supporting Information) (18).
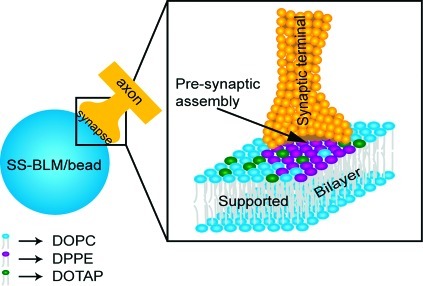
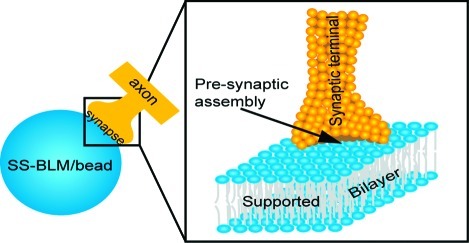
We thus suggest that the following mechanism plays a major role in the observed presynaptic assembly (Scheme 2). The presence of DPPE-enriched lipid domains on the SS-BLM/bead surface, together with the chemical specificity (through −NH2 groups), signals the axons that are already in contact with SS-BLM/beads (due to the presence of positively charged DOTAP in the membrane) to trigger presynaptic vesicle accumulation at the bead−axon contacts. Control experiments (when no domain formation was possible; DOPE case) support the possibility that such a mechanism is operative.
Scheme 2. Scheme (Not to Scale) Suggesting a Possible Mechanism of SS-BLM/Bead-Induced Presynaptic Assembly.
SS-BLM/beads first adher to axons via electrostatic attraction provided by the cationic lipid DOTAP (green head groups) distributed in the zwitterionic DOPC (blue head groups) matrix. The presence of DPPE (purple head groups) via chemical (through −NH2) and physical (lipid phase domains) interactions signals axons to form synapses in some fashion.
It has been shown previously that mechanical properties of membranes along with cytoskeletal rearrangements play a vital role in the biochemical reactions taking place at intermembrane junctions like synapses (11). Cytoskeletal networks, which link the cell cytoplasm to the outer plasma membrane leaflet, are responsible for stabilizing cell signaling events in neurons (13). The role of the actin filaments at the presynaptic site (19), especially at the early stages of synapse formation (3), has already been documented (Figure 4b confirms the presence of actin filaments at the SS-BLM-induced presynaptic site). The work of Liu et al. using model lipid membranes convincingly shows that an actin network can in fact induce formation of membrane domains or can stabilize the existing lipid domains (20). Lipid microdomains are also known to be present in the dendrites of hippocampal neurons in high abundances and are shown to be associated with several postsynaptic proteins (21). Given the fact that our SS-BLM offers coexisting lipid microdomains, it may allow for synchronization of all the above-mentioned crucial cellular processes (14,21). It is possible that axons could find their targets on the SS-BLM/beads due to their mechanical similarity to postsynaptic membranes. Following this, formation of stable presynaptic terminals could occur via signal transduction cascades that lead to reorganization of actin filaments that are responsible for the prestabilization during the “pathfinding” stages of early synapse formation. It is important to note that when the crucial individual lipid composition was absent (Figure SI.3, Supporting Information), events starting from the actin prestabilization (as in Figure 4b) do not occur. This further supports our hypothesis that the observed in vitro synapse formation is a result of certain signaling cascades that occur only in the presence of particular chemical and physical elements present on the artificial substrate.
In summary, we have reported a method to rationally design cationic SS-BLMs on silica beads for use as substrates for in vitro presynaptic assembly that recruit several important elements that are reported to be present in a presynaptic active zone. Though the exact molecular mechanism behind it is still not completely understood, we believe that this work is the first step in addressing the possible role of lipid membrane domains at synaptic sites in a purely in vitro configuration. The deliberate inclusion of individual lipids with known functionalities in the bilayer thus creates an artificial substrate that shows potential to be used in mechanistic studies as well as to study synaptogenesis (22)in vivo. The possibility of extending our SS-BLM approach to other geometries such as silica fibers may help to improve the ongoing attempts to create suitable surface matrices that mimic axon surfaces for their eventual artificial myelination. Moreover, combining neurologically relevant membrane proteins (1a), CNS specific receptors, or both onto SS-BLM scaffolds (9,11), which are more precisely tuned for their mechanical properties, would be a step toward functional induced synapse formation (23). Taken together, the biocompatibility and membrane tunability make these SS-BLM/beads a promising candidate for neuroengineering applications.
Methods
Lipids Used
The following lipids were from Avanti Polar Lipids: 1,2-dioleoyl-sn-glycero-3-phosphatidylcholine (DOPC), 1,2-dioleoyl-3-trimethylammonium propane (chloride salt) (DOTAP), 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine (DPPE), 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE), 1,2-disteroyl-sn-glycero-3-phosphatidylethanolamine-N-[biotinyl(poly(ethylene glycol)2000)] (ammonium salt) (DSPE−PEG2000−biotin), and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-[7-nitro-2-1,3-benzoxadiazol-4-yl] (ammonium salt) (DOPE−NBD). N-(6-Tetramethylrhodaminethiocarbamoyl)-1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine, triethylammonium salt (TRITC−DHPE) was purchased from Invitrogen (USA).
Preparation of SS-BLM/Beads
Silica beads (Bangs Laboratories) were diluted to 1:100 in PBS, washed twice in PBS by centrifugation, and then resuspended and incubated in 1 mL of PBS containing 0.05 mg/mL avidin overnight at 4 °C. The avidin-treated beads were then washed several times and resuspended in a final volume of 500 μL of PBS prior to incubation with the lipids.
Chloroform solutions of DOPC (1.5 mM, 75 μL), DOTAP (1.5 mM, 25 μL), and DSPE−PEG2000−biotin (0.6 mM, 5 μL) were mixed and dried in vacuum for 4 h under sterile conditions. The film was then hydrated using sterile PBS through rapid mixing followed by sonication in a bath sonicator for 3 min, which results in the formation of small unilamellar vesicles (SUVs).
The vesicle solution (500 μL) was then mixed with 500 μL of the avidin-coated silica beads dispersed in sterile PBS and incubated for 10 min with gentle shaking. The bead−vesicle solution was then mixed vigorously and sonicated for 1 min followed by centrifugation (12 000 rpm for 12 min), and the resulting pellet was then resuspended in PBS. The bilayer-coated beads were used within 1 week of preparation and added to cultures as described below.
Primary Cultures of Rat Hippocampal Neurons
Cultures of dissociated rat hippocampal neurons were prepared using a modification of a protocol described by Banker (25). Hippocampi were dissected from E17/18 embryos, treated with 0.25% trypsin at 37 °C followed by Dulbecco's modified Eagle medium (DMEM)−10% horse serum, and mechanically dissociated with a plastic Pasteur pipet. The dissociated neurons were plated at a density of (2.0−2.5) × 104 cm−1 on, unless otherwise stated, poly-d-lysine (Sigma) coated glass coverslips in serum-free neurobasal medium supplemented with l-glutamine and B-27. One-third of the medium was replaced every 2−3 days. All culture media were purchased from Gibco (Invitrogen).
Coculture with Silica Beads
Neurons were cultured to various stages of development (between 7 and 21 DIV) before the addition of beads. The lipid bilayer coated beads were suspended in sterile PBS, pH 7.4, and added dropwise to the neurons at a concentration of (1.0−1.5) × 105 beads/coverslip. The bead/cell coculture was incubated for 24 h in a humidified 5% CO2 atmosphere at 37 °C.
Immunocytochemistry
Cells were fixed with 4% paraformaldehyde in phosphate buffer (PB), pH 7.4, for 25 min, incubated in blocking solution (Tris-buffered saline (TBS), pH 7.4, containing 4% normal donkey serum (NDS) and 0.1% Triton-X100) for 30 min, and then in primary antibodies diluted in TBS containing 0.1% Triton-X100 and 0.5% normal donkey serum (NDS), overnight at 4 °C with gentle shaking. Cells were washed in TBS, incubated in Alexa-488/Alexa-543/Cy-5 (as appropriately) coupled secondary antibodies (in TBS-0.5%, NDS), washed 3× in TBS, and mounted on glass slides. Primary antibodies used were rabbit polyclonal anti-synaptophysin (Invitrogen, predilute; 1:10), mouse monoclonal anti-bassoon (Assay Designs, Ann Arbor, MI), and all secondary antibodies (species-specific, highly cross-adsorbed IgG) were purchased from Invitrogen and used at a dilution of 1:200. For actin labeling, Alexa 488−phalloidin (Molecular Probes) was used (1:50) in the secondary antibody dilution buffer. The stained samples (on glass coverslips) were mounted using GelTol on microscopic slides and sealed.
Confocal Microscopy
All the fluorescence images were obtained using a Zeiss LSM 510 META confocal microscope (Carl Zeiss AG, Germany) with λex 488 nm/λem LP > 505 nm or BP 505−550 IR, λex 543 nm/λem LP > 565 nm, and λex 633 nm/λem LP > 685 nm optical combinations appropriately for neuron/bead cultures and lipid bilayers in different experiments. The images were obtained with a 63× oil immersion objective on an inverted microscope along with the corresponding brightfield (diffusion interference contrast or DIC) image.
Cryo-TEM
Five microliters of a solution of silica beads (both uncoated and lipid coated) was added to copper Quantifoil R2/2 grids (Electron Microscopy Sciences, Hatfield, PA). Samples were blotted and frozen hydrated by plunging into a bath of liquid ethane slush (26). They were stored under liquid nitrogen temperature (−78 °C) until transfer to a 626 single tilt cryotransfer system (Gatan Inc.) and observed with a FEI G2 F20 cryo-STEM microscope operated at 200 kV (FEI, Inc.). Images were recorded under low-dose conditions on a Gatan Ultrascan 4k × 4k digital (CCD) camera system camera at a nominal magnification of 80× at a defocus level of 2 μm. Images of silica beads uncoated and coated with lipids were taken for direct comparison under the exact same conditions of magnification and defocus.
Acknowledgments
The authors thank Dr. A. S. Dhaunchak (MNI & H, McGill University) for helpful discussions and Lucas Medwell for assistance in the laboratory.
Supporting Information Available
Additional data including extended figures. This material is available free of charge via the Internet at http://pubs.acs.org.
G.G, D.R.C., and R.B.L designed the experiments. G.G. and I.R. performed the experiments. P.T., A.L.L., and W.B. supported the experiments. G.G., I.R., D.R.C., and R.B.L. analyzed the experimental results. G.G. and R.B.L. wrote the manuscript, and all the authors contributed to the editing of the manuscript.
This work is supported by a grant (no. RMF-79028) from the Regenerative Medicine and Nanomedicine Initiative of the Canadian Institutes of Health Research (CIHR) to R.B.L and D.R.C and a CIHR grant (no. RMF-86693) to I.R. I.R is recipient of a CIHR New Investigator Award. The McGill Program in NeuroEngineering is supported by CIHR and the Ministry of Industry of Canada (a Centre of Excellence in Commercialization and Research Award to the MNI&H), Rio Tinto Alcan, and The Molson Foundation.
Supplementary Material
References
- a Shapiro L.; Love J.; Colman D. R. (2007) Adhesion molecules in the nervous system: Structural insights into function and diversity. Annu. Rev. Neurosci. 30, 451–474. [DOI] [PubMed] [Google Scholar]; b Park D. H.; Eve D. J.; Borlongan C. V.; Klasko S. K.; Cruz L. E.; Sanberg P. R. (2009) From the basics to application of cell therapy, a steppingstone to the conquest of neurodegeneration: A meeting report. Med. Sci. Monit. 15, RA23–RA31. [PubMed] [Google Scholar]; c Agrawal S.; Schaffer D. V. (2005) In situ stem cell therapy: Novel targets, familiar challenges. Trends Biotechnol. 23, 78–83. [DOI] [PubMed] [Google Scholar]
- Pluchino S.; Zanotti L.; Brini E.; Ferrari S.; Martino G. (2009) Regeneration and repair in multiple sclerosis: The role of cell transplantation. Neurosci. Lett. 456, 101–106. [DOI] [PubMed] [Google Scholar]; Sharp J.; Keirstead H. S. (2009) Stem cell-based cell replacement strategies for the central nervous system. Neurosci. Lett. 456, 107–111. [DOI] [PubMed] [Google Scholar]; Tatard V. M.; Menei P.; Benoit J. P.; Montero-Menei C. N. (2005) Combining polymeric devices and stem cells for the treatment of neurological disorders: A promising therapeutic approach. Curr. Drug Targets 6, 81–96. [DOI] [PubMed] [Google Scholar]; Silva G. A. (2006) Neuroscience nanotechnology: Progress, opportunities and challenges. Nat. Rev. Neurosci. 7, 65–74. [DOI] [PubMed] [Google Scholar]; Orive G.; Anitua E.; Pedraz J. L.; Emerich D. F. (2009) Biomaterials for promoting brain protection, repair and regeneration. Nat. Rev. Neurosci. 10, 682–692. [DOI] [PubMed] [Google Scholar]
- Lucido A. L., Sanchez F. S., Thostrup P., Kwiatkowski A. V., Ortiz S. L., Gopalakrishnan G., Liazoghli D., Belkaid W., Lennox R. B., Grutter P., Garner C. C., and Colman D. R. (2009) Rapid assembly of functional presynaptic boutons triggered by adhesive contacts J. Neurosci. 29, 12449−12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burry R. W. (1980) Formation of apparent presynaptic elements in response to poly-basic compounds. Brain Res. 184, 85–98. [DOI] [PubMed] [Google Scholar]; Burry R. W. (1983) Postnatal rat neurons form apparent pre-synaptic elements on polylysine-coated beads in vivo. Brain Res. 278, 236–239. [DOI] [PubMed] [Google Scholar]
- Cevc G., Marsh D. (1987) Phospholipid Bilayers: Physical Principles and Models, Wiley-VCH, New York. [Google Scholar]; Gennis R. B. (1989) Biomembranes: Molecular Structure and Function, Springer-Verlag, New York. [Google Scholar]; Mouritsen O. G. (2005) Life- as a Matter of Fat. The Emerging Science of Lipidomics, Springer Verlag, Heidelberg. [Google Scholar]
- Gopalakrishnan G.; Rouiller I.; Colman D. R.; Lennox R. B. (2009) Supported bilayers formed from different phospholipids on spherical silica substrates. Langmuir 25, 5455–5458. [DOI] [PubMed] [Google Scholar]
- Troutier A.-L.; Ladavière C. (2007) An overview of lipid membrane supported by colloidal particles. Adv. Colloid Interface Sci. 133, 1–21. [DOI] [PubMed] [Google Scholar]; Mornet S.; Lambert O.; Duguet E.; Brisson A. (2005) The formation of supported lipid bilayers on silica nanoparticles revealed by cryoelectron microscopy. Nano Lett. 5, 281–285. [DOI] [PubMed] [Google Scholar]
- Terrettaz S.; Ulrich W.-P.; Guerrini R.; Verdini A.; Vogel H. (2001) Immunosensing by a synthetic ligand-gated ion channel. Angew. Chem., Int. Ed. 40, 1740–1743. [PubMed] [Google Scholar]
- Baksh M. M.; Dean C.; Pautot S.; DeMaria S.; Isacoff E.; Groves J. T. (2005) Neuronal activation by GPI-linked neuroligin-1 displayed in synthetic lipid bilayer membranes. Langmuir 21, 10695–10698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Gopalakrishnan G.; Danelon C.; Izewska P.; Prummer M.; Bolinger P.-Y.; Geissbühler I.; Demurtas D.; Dubochet J.; Vogel H. (2006) Multifunctional lipid/quantum dot hybrid nanocontainers for controlled targeting of live cells. Angew. Chem., Int. Ed. 45, 5478–5483. [DOI] [PubMed] [Google Scholar]; b Thid D.; Holm K.; Eriksson P. S.; Ekeroth J.; Kasemo B.; Gold J. (2008) Supported phospholipid bilayers as a platform for neural progenitor cell culture. J. Biomed. Mater. Res. 84A, 940–953. [DOI] [PubMed] [Google Scholar]
- Groves J. T. (2005) Molecular organization and signal transduction at intermembrane junctions. Angew. Chem., Int. Ed. 44, 3524–3538. [DOI] [PubMed] [Google Scholar]
- a Felgner P. L.; Gadek T. R.; Holm M.; Roman R.; Chan H. W.; Wenz M.; Northrop J. P.; Ringold G. M.; Danielsen M. (1987) Lipofection- a highly efficient, lipid-mediated DNA-transfection procedure. Proc. Natl. Acad. Sci. U.S.A. 84, 7413–7417. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Wrobel I.; Collins D. (1995) Fusion of cationic liposomes with mammalian-cells occurs after endocytosis. Biochim. Biophys. Acta-Biomembranes 1235, 296–304. [DOI] [PubMed] [Google Scholar]; c Simberg D.; Weisman S.; Talmon Y.; Barenholz Y. (2004) DOTAP (and other cationic lipids): Chemistry, biophysics, and transfection. Crit. Rev. Ther. Drug Carrier Syst. 21, 257–317. [DOI] [PubMed] [Google Scholar]
- Janmey P. A. (1998) The cytoskeleton and cell signaling: component localization and mechanical coupling. Physiol. Rev. 78, 763–781. [DOI] [PubMed] [Google Scholar]; Sobue K.; Kanda K. (1989) Alpha-actinins, calspectin (brain spectrin or fodrin), and actin participate in adhesion and movement of growth cones. Neuron 3, 311–319. [DOI] [PubMed] [Google Scholar]; Gomez T. M.; Zheng J. Q. (2006) The molecular basis for calcium-dependent axon pathfinding. Nat. Rev. Nerosci. 7, 115–125. [DOI] [PubMed] [Google Scholar]
- Ziv N. E.; Garner C. C. (2004) Cellular and molecular mechanisms of presynaptic assembly. Nat. Rev. Neurosci. 5, 385–399. [DOI] [PubMed] [Google Scholar]
- a Munro S. (2003) Lipid rafts: Elusive or illusive?. Cell 115, 377–388. [DOI] [PubMed] [Google Scholar]; b Foster L. J.; De Hoog C. L.; Mann M. (2003) Unbiased quantitative proteomics of lipid rafts reveals high specificity for signaling factors. Proc. Natl. Acad. Sci. U.S.A. 100, 5813–5818. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Pike L. J. (2003) Lipid rafts: Bringing order to chaos. J. Lipid Res. 44, 655–667. [DOI] [PubMed] [Google Scholar]; d Simons K.; Toomre D. (2000) Lipid rafts and signal transduction. Nat. Rev. Mol. Cell. Biol. 1, 31–39. [DOI] [PubMed] [Google Scholar]; e Golub T.; Wacha S.; Caroni P. (2004) Spatial and temporal control of signaling through lipid rafts. Curr. Opin. Neurobiol. 14, 542–550. [DOI] [PubMed] [Google Scholar]
- Wen Z.; Zheng J. Q. (2006) Directional guidance of nerve growth cones. Curr. Opin. Neurobiol. 16, 52–58. [DOI] [PubMed] [Google Scholar]
- a Bagatolli L. A.; Gratton E. (2000) Two photon fluorescence microscopy of coexisting lipid domains in giant unilamellar vesicles of binary phospholipid mixtures. Biophys. J. 78, 290–305. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Korlach J.; Schwille P.; Webb W. W.; Feigenson G. W. (1999) Characterization of lipid bilayer phases by confocal microscopy and fluorescence correlation spectroscopy. Proc. Natl. Acad. Sci. U.S.A. 96, 8461–8466. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Hac A. E.; Seeger H. M.; Fidorra M.; Heimburg T. (2005) Diffusion in two-component lipid membranes - a fluorescence correlation spectroscopy and monte carlo simulation study. Biophys. J. 88, 317–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- In order to further support our suggested mechanism and to exclude other possible mechanisms, we tested whether any membrane fusion related process plays a role. This is because PEG molecules are known to have fusogenic effects when present in either of the membranes that are undergoing membrane fusion (10a,24). The results imply that SS-BLM/beads made up of the fusogenic DOPC/DOTAP/DPPE−PEG2000 (25:25:50) mixture (10a) were negative for synaptophysin labeling (Figure SI.6, Supporting Information). This suggests that membrane fusion events may be excluded. Though the presence of DOTAP together with DPPE could arguably lead to membrane fusion, lipid exchange, and endocytosis mediated mechanisms (12c), our control experiments suggest that other types of mechanisms are more likely operative. This is further supported by the presence of intact bilayers on the SS-BLMs after 24 h of incubation with hippocampal neurons (Figure SI.7, Supporting Information). Being supported on a solid support could very well reduce the possibility of fusion/endocytosis of the SS-BLM/beads.
- Cingolani L.; Goda Y. (2008) Nat. Rev. Neurosci. 9, 344–356. [DOI] [PubMed] [Google Scholar]; Dillon C.; Goda Y. (2005) Actin in action: The interplay between the actin cytoskeleton and synaptic efficacy. Annu. Rev. Neurosci. 28, 25–55. [DOI] [PubMed] [Google Scholar]; Dent E.; Gertler F. B. (2003) The actin cytoskeleton: Integrating form and function at the synapse. Neuron 40, 209–227.14556705 [Google Scholar]; Sankaranarayanan S.; Alturi P.; Ryan T. (2003) Actin has a molecular scaffolding, not propulsive, role in presynaptic function. Nat. Neurosci. 6, 127–135. [DOI] [PubMed] [Google Scholar]
- Liu A. P.; Fletcher D. A. (2006) Actin polymerization serves as a membrane domain switch in model lipid bilayers. Biophys. J. 91, 4064–4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hering H.; Lin C.-C.; Sheng M. (2003) Lipid rafts in the maintenance of synapses, dendritic spines, and surface AMPA receptor stability. J. Neurosci. 23, 3262–3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson D. L.; Colman D. R.; Huntley G. W. (2001) Molecules, maps and synapse specificity. Nat. Rev. Neurosci. 2, 899–909. [DOI] [PubMed] [Google Scholar]
- Dustin M. L.; Colman D. R. (2002) Neural and immunological synaptic relations. Science 298, 785–789. [DOI] [PubMed] [Google Scholar]
- Lentz B. R. (1994) Polymer-induced membrane-fusion-potential mechanism and relation to cell-fusion events. Chem. Phys. Lipids 73, 91–106. [DOI] [PubMed] [Google Scholar]; Hui S. W.; Kuhl T. L.; Guo Y. Q.; Israelachvili J. (1999) Use of poly(ethylene glycol) to control cell aggregation and fusion. Colloids Surf., B 14, 213–222. [Google Scholar]
- Goslin K., Asmussen H., Banker G. (1998) Culturing Nerve Cells (Banker G., Goslin K., Ed.), 2nd ed., pp 339−370, MIT-Press, Cambridge, MA. [Google Scholar]
- Dubochet J.; Adrian M.; Chang J. J.; Homo J. C.; Lepault J.; McDowall A. W.; Schultz P. (1988) Cryo-electron microscopy of vitrified specimens. Q. Rev. Biophys. 21, 129–228. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.