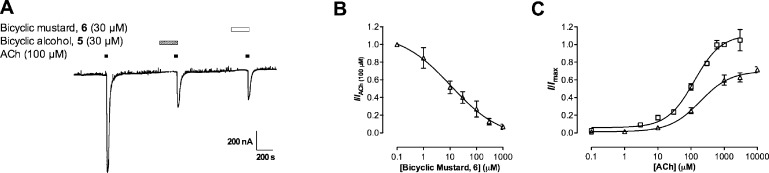
Figure 7.
(A) Trace showing the effect of ACh (100 μM; duration indicated by black bar) and ACh in the presence of bicyclic alcohol 5 (30 μM; duration indicated by hatched bar) and bicyclic mustard 6 (30 μM; duration indicated by white bar). Both bicyclic alcohol 5 and bicyclic mustard 6 were preincubated for 3 min before coapplying with ACh. (B) Concentration-inhibition curve for bicyclic mustard 6 (○) in the presence of ACh (100 μM) at α4β2 nAChR. The IC50 value was 10.9 μM (95% CI: 2.40−49.8) and was not statistically different (Student’s t test; p > 0.05) to bicyclic alcohol 5 at α4β2 nAChR under the same conditions (Figure 3). (C) Concentration response curves for ACh alone (◻) and ACh in the presence of bicyclic mustard 6 (30 μM) with a 3 min preincubation (Δ) at α4β2 nAChRs. Bicyclic mustard 6 was a noncompetitive antagonist at α4β2 nAChRs (represented by a significant drop in Imax and no significant rightward shift of the ACh EC50 (Table 1).