Fig. 2.
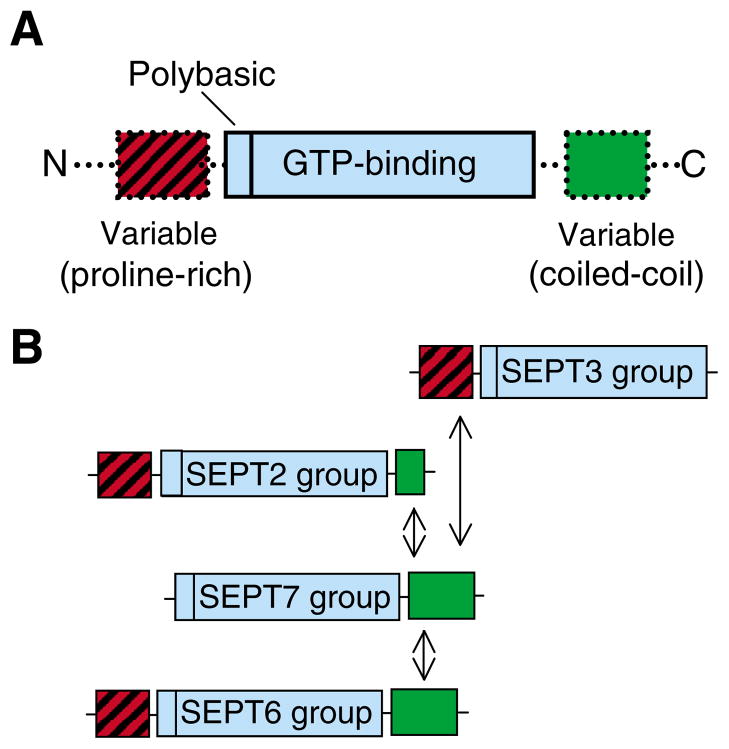
(A) Septin structure. All mammalian septins consist of a polybasic region and a GTP-binding domain of the P-loop superfamily of GTPases. Their N- and C-terminal regions vary in length and amino acid composition, and contain proline-rich (red) and α-helical coiled-coil (green) domains. (B) Assembly and classification of mammalian septins based on amino acid similarity (Kartmann and Roth, 2001; Kinoshita, 2003; Hall et al., 2005). Septins are classified into four groups: SEPT3 (SEPT3, SEPT9 and SEPT12), SEPT2 (SEPT2, SEPT1, SEPT4 and SEPT5), SEPT7 (SEPT7 and SEPT13) and SEPT6 (SEPT6, SEPT8, SEPT10 and SEPT11). Note that proteins of the SEPT7 group were recently classified under the SEPT2 group (Hall et al., 2005). Proteins of the SEPT3 group lack C-terminal coiled-coil domains, and the SEPT2 group contains a shorter coiled-coil region than SEPT6. Septins bind one another through their coiled-coil domains to form hetero-oligomers and hetero-polymers (Sheffield et al., 2003). SEPT9 isoforms (SEPT3 group) associate with other septins through their N-terminal domain (Nagata et al., 2004).