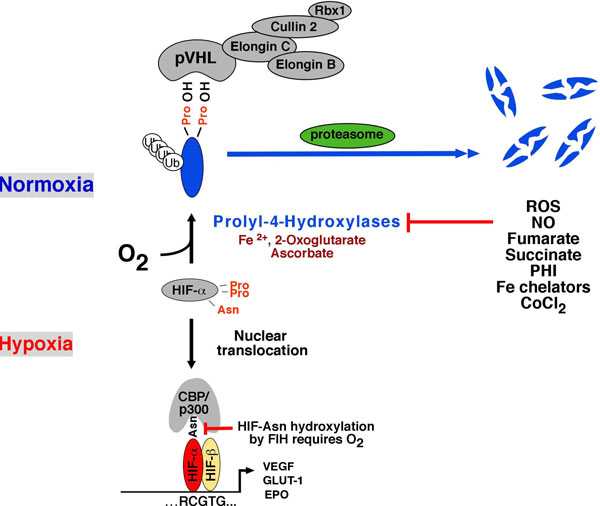
Figure 1.
Overview of PHD/HIF signaling. Under normoxia, both HIF-1α and HIF-2α are hydroxylated by prolyl-4-hydroxylases and are targeted for proteasomal degradation by the von Hippel-Lindau (pVHL)-E3 ubiquitin ligase complex (shown are key components of this complex). Binding to prolyl-hydroxylated HIF-α occurs at the β-domain of pVHL, which spans amino acid residues 64 - 154. The C-terminal α-domain links the substrate recognition component pVHL to the E3 ubiquitin ligase via elongin C. When prolyl-4-hydroxylation is inhibited (e.g. by hypoxia, ROS), HIF-α subunits are stabilized and translocate to the nucleus where they heterodimerize with ARNT. HIF-α/ARNT heterodimers bind to the HIF consensus-binding site, RCGTG, resulting in increased expression of target genes. Factor-inhibiting-HIF (FIH) is a dioxygenase that modulates transcriptional cofactor recruitment (CBP/p300) via asparagine (Asn) hydroxylation of the HIF-α carboxy-terminal transactivation domain. In addition to ROS, nitric oxide, Krebs cycle metabolites succinate and fumarate, cobalt chloride and iron chelators such as desferrioxamine inhibit HIF prolyl-4-hydroxylases in the presence of oxygen. Abb.: CoCl2, cobalt chloride; Fe2+, ferrous iron; NO, nitric oxide; PHI, prolyl-4-hydroxylase inhibitors (structural 2-oxoglutarate analogs); ROS, reactive oxygen species; ub, ubiquitin.