Figure 1.
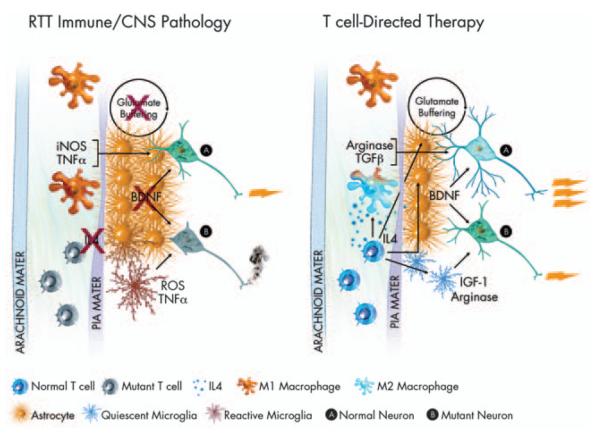
Hypothesis—immune function in RTT. RTT is most often caused by a mutation in the MECP2 gene. As MECP2 is X-linked, because of X-inactivation, some cells in afflicted females express the mutant form of the gene, whereas others do not. In the above illustration, mutant T cells are shown in an inactive or anergic state. Inactive T cells produce low levels of cytokines, in particular IL4. Loss of T-cell modulation of meningeal immunity has been shown to lead to an M1 meningeal myeloid skew. M1 myeloid cells produce high levels of pro-inflammatory neurodestructive cytokines (iNOS, TNF-α)asin turn do reactive microglia. Reactive astrocytes buffer glutamate at reduced levels, and produce substantially diminished levels of BDNF. All these factors combined lead to an environment in CNS impoverished in critical neuronal growth factors, leading to massive dysfunction in mutant neurons (B), and diminished function in normal neurons (A). Amelioration of T-cell function could return homeostasis to the meningeal immune milieu. Normal levels of IL4 would push meningeal myeloid cells to an M2 fate, encouraging production of neuroprotective factors such as TGFβ and arginase. T-cell-derived interferon-γ (not shown) and IL4 would also support improved glutamate buffering and improved BDNF production by astrocytes as well as production of arginase and IGF-1 by microglia. An improved meningeal and glial environment would lead to strong support for normal neurons (A) and mutant neurons (B) alike, thus improved neural function overall and amelioration of RTT. Several immune-related genes (e.g. MHC) have been connected to ASD in linkage analysis studies, thus some ideas expressed in this paradigm can be generalized.