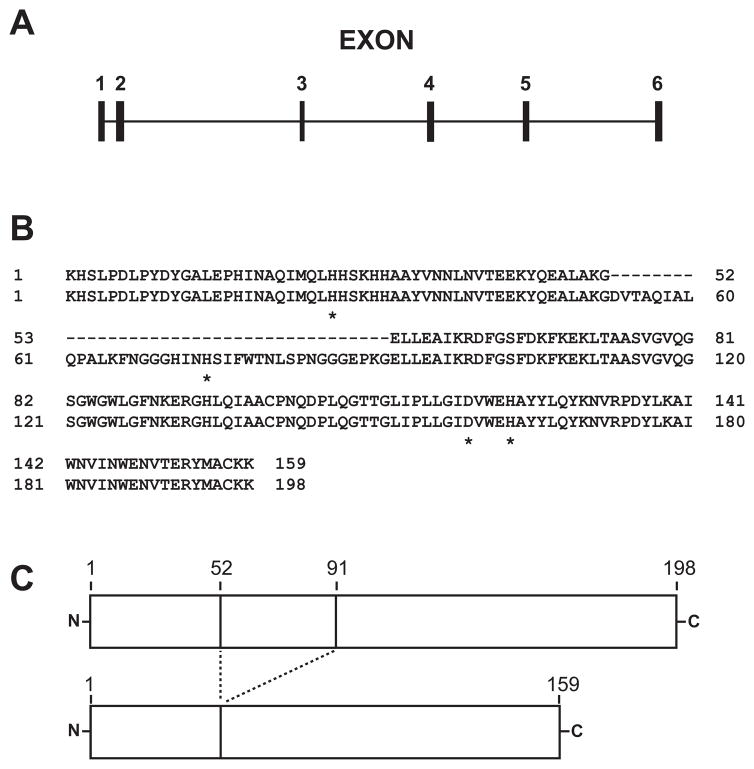
Fig. 1.
Organization of the human SOD2 pre-mRNA and comparison of hMnSOD isoforms A and B. (A) Predicted exon/intron organization of the human SOD2 pre-mRNA. Exon 3 is retained in the variant 1 splicing product, encoding hMnSOD isoform A, but is eliminated from the variant 3 splicing product, encoding hMnSOD isoform B. (B) Alignment of mature protein sequences for hMnSOD isoforms A (bottom) and B (top). Residues that serve as metal ligands in hMnSOD isoform A are indicated by an asterisk (*). (C) Comparison of hMnSOD isoform A (top) and B (bottom) mature proteins, illustrating the different polypeptide lengths.