Abstract
Purpose
To establish a relationship between hypoxic stress and the expression of ΔNp63α in an established rabbit contact lens model and in cultured corneal epithelial cells.
Methods
New Zealand White rabbits were fit in one eye with either a non-oxygen transmissible or hyper oxygen permeable rigid contact lens for 24 hours of wear; the contralateral eye was used as a control. All rabbits underwent a bilateral nictitating membranectomy to facilitate lens retention. ΔNp63α expression was analyzed by immunofluorescence and western blot. Telomerase-immortalized human corneal epithelial cells (hTCEpi) were grown in serum-free media and treated with the hypoxia mimetic cobalt chloride to simulate hypoxia for 6 (short term) or 24 (prolonged) hours. Transcriptional activity and protein levels were assessed using luciferase reporter assays, RT-PCR, and western blot. Cell viability was assessed by live/dead assay.
Results
Compared to the non-lens wearing eye, 24 hours of non-oxygen transmissible lens wear in vivo decreased ΔNp63α protein levels in both the limbal and central corneal epithelium; this decrease was not found in the hyper oxygen transmissible lens group. In hTCEpi cells in vitro, hypoxia increased the activity of the ΔN promoter, but reduced levels of ΔNp63α mRNA after 24 hours of prolonged culture. Similarly, ΔNp63α expression levels were unaffected from short term exposure, but decreased after 24 hours. Live/dead assay confirmed the presence of viable cells following CoCl2 treatment at 6 and 24 hour time points. Cells treated for 24 hours were viable but were smaller and rounded with signs of membrane blebbing, consistent with early stages of apoptosis.
Conclusions
Hypoxic stress induced by either prolonged wear of a non-oxygen transmissible lens in vivo or hypoxic-mimic conditions by cobalt chloride in vitro down regulates ΔNp63α in the corneal epithelium. The loss of ΔNp63α in response to hypoxic stress may contribute to the disruption of normal renewal mechanisms reported with low oxygen transmissible contact lens wear and prolonged eyelid closure.
Keywords: ΔNp63, epithelium, hypoxia, contact lens
Introduction
Homeostatic renewal of the corneal epithelium is defined as the dynamic coordination between basal cell proliferation, vertical terminal differentiation and surface epithelial desquamation, and is essential for maintenance of the 5–7 cell layered epithelial sheet.1 All forms of contact lens wear impede the natural renewal process of the epithelium, producing a thinned, stagnant epithelial surface, potentially predisposing the cornea to infection by invading pathogens.2–5 Clinical and bench studies have examined the effects of contact lenses on renewal mechanism in the corneal epithelium.6–8 The cumulative results from this work have shown that contact lens wear perturbs the normal biology of the corneal surface through the inhibition of surface cell desquamation and a reduction in basal cell proliferation.9–13 Importantly, these effects have been shown to be mediated in part by lens oxygen transmissibility.4, 14, 15
In a standardized rabbit contact lens model, large diameter rigid gas permeable lens wear has been shown to induce corneal epithelial surface damage quantitated by confocal microscopy that is directly proportional to the oxygen transmissibility of the lens material, and has been further confirmed by scanning electron microscopy and a concomitant increase in tear lactate dehydrogenase activity levels released from damaged surface cells.16, 17 Similarly, varying degrees of hypoxia induced by rigid lens wear in the rabbit have been shown to correspond with a decrease in BrdU-labeled daughter cells in the basal cell layer of the epithelium, indicating a repression in basal cell mitosis in response to lens-induced hypoxia. In the absence of lens wear, prolonged eyelid closure in the rabbit model has also been shown to induce a chronic state of hypoxia, similar to low oxygen transmissible contact lenses, and is associated with a slowing of normal homeostatic renewal mechanisms and alterations in the integrity of the corneal surface.14,18
Recently, we have investigated the potential role of ΔNp63 as a key regulator of renewal mechanisms in the corneal epithelium.19 ΔNp63, regarded as the master regulator for stratified epithelial development, is highly expressed in the regenerative, basal layer of stratified epithelia.20–23 In the corneal epithelium, high levels of the ΔNp63 splice variant ΔNp63α, have been shown to localize to the limbal stem cell compartment as well as early transient amplifying (TA).24 Clonal analysis of ΔNp63α expression in subconfluent primary cultures have further demonstrated ΔNp63α expression to be highest in holoclones, which are presumably early TA cells endowed with the greatest proliferative capacity.25 In vitro studies in our laboratory have shown that ΔNp63α is highly expressed throughout the log growth phase and rapidly down-regulated upon cellular confluence and during calcium-induced differentiation.19 In contrast to earlier reports however, ΔNp63α has been shown to persist throughout basal cells in the central corneal epithelium and is completely lost only in the pre-desquamative outermost surface cell layer, suggesting additional roles for ΔNp63α in regulating cell survival.26
In the current study, in efforts to elucidate a role for ΔNp63α in mediating homeostatic renewal in the corneal epithelium, we utilized the well-established rabbit contact lens model. The results show that ΔNp63α expression is decreased during prolonged hypoxic contact lens wear in vivo. We further confirmed that both hypoxia and the well-established hypoxia-mimetic cobalt chloride decreased ΔNp63α in a telomerase-immortalized corneal epithelial cell line in vitro. The loss of ΔNp63α is a direct result of long-term hypoxia-induced transcriptional down regulation and is associated with loss of cell viability. These findings support a role for ΔNp63α in homeostatic maintenance of the central corneal epithelium and suggest that the loss of ΔNp63α that occurs during prolonged hypoxia induced by contact lens wear may explain corneal epithelial damage reported in in vivo animal studies.
Methods
Cell Culture
Human telomerase-immortalized corneal epithelial cells (hTCEpi) were initially isolated and thereafter routinely maintained in serum-free keratinocyte growth media (KGM-2, Clonetics-BioWhittaker) containing 0.15 mM calcium and supplemented with 0.4% bovine pituitary extract, 0.1% human epidermal growth factor, 0.1% insulin, 0.1% hydrocortisone, 0.1% transferrin, 0.1% epinephrine and 0.1% gentamicin sulfate amphotericin B as previously described.27 Cells were subcultured on T75 tissue culture flasks (Falcon Labware; BD Biosciences, Bedford, MA), incubated at 37 °C in 5% CO2. and passaged every 4 – 5 days. To achieve hypoxic conditions, cells were treated with the hypoxia-mimetic cobalt chloride (150 μM CoCl2, Sigma, St. Louis, MO) for 6 or 24 hours. Cobalt chloride is commonly used to mimic hypoxic conditions in epithelial cultures and is associated with induction of hypoxia-inducible factor 1 alpha (HIF-1α).28– 30 To confirm hypoxic effects of CoCl2, cells were also incubated in sealed plastic chambers containing 10% O2, 85% N2 and 5% CO2 or 95% N2 and 5% CO2 for 24 hours. Parallel cultures were grown under normoxic conditions. To establish exact concentrations of relative gases, a custom gas cylinder was used and directly purged through a Whatman vent disposable filter into the sealed chamber and maintained for the duration of the experiment.
Animals
Thirteen New Zealand white rabbits (2.5 – 3.5 kg body weight) were used in this study. All animals were treated humanely in accordance with the ARVO statement for the use of animals in ophthalmic and vision research. To permit contact lens retention, prior to lens fitting, all rabbits underwent a standardized partial nictitating membranectomy under anesthesia as previously described.12 All animals were given a 1 week recovery period prior to initiating lens wear. One eye of each rabbit was fit using fluorescein with either a non-oxygen transmissible polymethylmethacrylate (PMMA) or a hyper-oxygen transmissible (Menicon Z, Menicon Co, Ltd., Nagoya, Japan) rigid lens. For both lens materials, the lens overall diameter was 14.0 mm and base curves ranged from 7.60 – 8.20 mm. Oxygen transmissibility of test lenses is detailed in Table 1. For all experiments, the contralateral eye served as a control. Following 24 hours of lens wear, lenses were carefully removed and animals were euthanized by an intravenous injection of 120 mg/kg sodium pentobarbital (Sleepaway, Fort Dodge, IA).
Table 1.
Oxygen transmissibility of test lenses.
| Lens Type | Material | Dk* | Dk/t** | EOP*** |
|---|---|---|---|---|
| Non-oxygen transmissible lens | Polymethylmethacrylate (PMMA) | 0 | 0 | 0 |
| Hyper-oxygen transmissible lens | Tisilfocon A | 146 | 97 | 19.13 |
Dk: oxygen permeability coefficient (cm/sec)(mL O2/mL mmHg) × 10−11
Dk/t: oxygen permeability/lens thickness
EOP: equivalent oxygen percentage; normal at sea level under open eye conditions is 21%.
Immunofluorescence
For immunofluorescence studies, excised rabbit corneal tissue was fixed in RNase free 1% paraformaldehyde (Electron Microscopy Sciences, Fort Washington, PA) in phosphate buffered saline (PBS), embedded in tissue embedding medium (Leica Instruments GmbH, Nussloch, Germany) and snap frozen in liquid nitrogen for cryostat sectioning. Cryostat-sectioned tissue was permeabilized with cold acetone (−20 °C) and washed with PBS. Tissue sections were blocked with 10% donkey serum for 30 minutes at 37 °C and incubated overnight in an anti-p63 mouse monoclonal antibody (clone 4A4, Sigma-Aldrich, St. Louis, MO) at 37 °C. Samples were subsequently washed in PBS and stained with a FITC-conjugated secondary donkey anti-mouse IgG (Jackson ImmunoResearch, West Grove, PA) for one hour at 37 °C. Nuclei were counterstained with propidium iodide (PI, Sigma, St. Louis, MO). All samples were mounted on slides using a 50% v/v solution of glycerol-PBS and imaged on a Leica SP2 laser scanning confocal microscope (Leica Microsystems, Heidelberg, Germany) using a 63x water objective.
Protein Extraction and Western Blotting
For protein extraction from the rabbit cornea, eyes were enucleated and a 6.0 mm trephine was used to separate the central/mid-peripheral cornea from the peripheral cornea/limbus. Epithelium was then mechanically removed using a No.11 scalpel blade (Lance, Sheffield, England) and placed in 100 μl lysis buffer. For protein extraction from hTCEpi cells in vitro, cells were lysed directly in the 6-well culture dish using 0.4 ml lysis buffer. All cells were lysed in radioimmunoprecipitation buffer (RIPA) buffer containing a protease inhibitor cocktail tablet (Complete-Mini, Roche Diagnostics, Indianapolis, IN) on ice for 10 minutes. Lysates were snap frozen in liquid nitrogen, vortexed and briefly centrifuged to remove the precipitate. All lysates were boiled for 5 minutes in 4x sample buffer pH 6.8 containing 0.25 M tris, 8% lauryl sulfate, 40% glycerol, 20% mercaptoethanol and 0.04% bromophenol blue, resolved on a 7.5% SDS gel (rabbit studies) or a 4–15% precast linear gradient polyacrylamide gel (cell culture studies, Bio-rad, Hercules, CA) and subsequently transferred to a nitrocellulose membrane. Membranes were blocked in 5% non-fat milk for 1 hour at room temperature and blotted using mouse monoclonal antibodies: anti-p63 clone 4A4 (Sigma-Aldrich, St. Louis, MO) and β-actin (Sigma-Aldrich, St. Louis, MO) overnight at 4 °C. Following a 1 hour incubation with an anti-mouse secondary antibody (Amersham Biosciences, Piscataway, NJ), protein was visualized using ECL Plus Detection Reagents (Amersham Biosciences, Piscataway, NJ) and imaged on a Typhoon Variable Mode Imager (Amersham Biosciences, Piscataway, NJ). Protein quantitation was performed using ImageQuant Software (Amersham Biosciences, Piscataway, NJ).
Dual Luciferase Reporter Assay
For assessment of ΔNp63 promoter activity, a luciferase reporter construct consisting of nucleotides −1584 to +61 of the ΔNp63 promoter cloned into the pGL3-basic vector (−1584Luc) was used. The pGL3-basic vector served as an empty vector control. Both the ΔNp63 promoter construct and the pGL3-basic vector were a generous gift from Dr. Satrajit Sinha, State University of New York at Buffalo. To account for variations in transfection efficiency and for normalization of luciferase expression, the pRenilla luciferase vector driven by the thymidine kinase promoter (pRL-TK, Promega, Madison, WI) was co-transfected with either the −1584Luc or pGL3-basic. All plasmids were transformed using NEB 5-alpha Competent E.coli (New England Biolabs, Ipswich, MA) and prepared using Qiagen mini and maxi-prep kits (Valencia, CA), for screening and transfection, respectively. Plasmids were sequencing confirmed at the McDermott Center for Growth and Development at UT Southwestern Medical Center, Dallas, Texas. hTCEpi cells were seeded onto plastic 24 well plates and grown to 70–80% confluence. Cells were transfected with 0.5 μg pGL3-basic or −1584luc and 0.3 μg pRL-TK plasmid DNA per well using TransIT-2020 transfection reagent (Mirus Bio, Madison, WI) diluted in MEM (Cellgro, Manassas, VA). Six hours post-transfection, media was changed to KGM-2 and cells were incubated in cobalt chloride for 6 or 24 hours. Non-treated cells were used as controls. Luciferase activity was measured using a Dual Luciferase Assay (Promega, Madison, Wisconsin) according to manufacturer instructions. Briefly, cells were washed with PBS and lysed using 250 μl of passive lysis buffer. 20 μl of cell lysates were transferred to a white opaque 96-well plate (Fisher Scientific, Pittsburg, PA). Firefly Luciferase was quantified following addition of luciferase assay reagent II and Renilla Luciferase was quantified following addition of Stop and Glo reagent. Luciferase expression was assessed using a Synergy HT Multi-Detection Microplate Reader (BioTek Instruments, Winooski, VT).
Semi-quantitative RT-PCR
Semi-quantitative RT-PCR was used to determine mRNA levels for ΔNp63α under normal and hypoxic conditions. hTCEpi cells were seeded in plastic 6 well culture dishes and grown to 70–80% confluence. Cells were incubated in cobalt chloride for 6 or 24 hours. Non-treated cells grown in parallel were used as controls. RNA was isolated using RNA Stat60 (TelTest, Fisher Scientific, Houston, TX) according to manufacturer instructions. After air-drying, RNA was re-suspended in RNA storage solution (Invitrogen, Carlsbad, CA) and concentration was measured using a SmartSpec 3000 spectrophotometer (Bio-Rad, Hercules, CA). Reverse transcription and PCR were performed using the SuperScript III One-step PCR system (Invitrogen, Carlsbad, CA) using 1 μg of total RNA. Isoform-specific primers were used to amplify ΔNp63α, β, and γ as previously reported.25 Primers were synthesized by Integrated DNA Technologies (Coralville, IA). Sequences and expected product size are listed in Table 2. β-actin was used as a house keeping gene. Reaction conditions were as follows: after an initial 30 minute incubation at 55°C for cDNA synthesis, PCR amplification included an initial denaturation cycle at 94°C for 2 minutes, followed by 30 cycles at 94.0°C for 15 seconds, 56°C for 30 seconds and 68.0°C for 2 minutes, and a final extension at 68.0°C for 5 minutes. Optimal cycle number was empirically determined. PCR products were diluted 1:5 in 10X Blue Juice (Invitrogen, Carlsbad, CA) and electrophoresed through a 1.25% ethidium bromide stained agarose gel. Resulting bands were visualized using a Typhoon Variable Mode Imager (Amersham Biosciences, Piscataway, NJ).
Table 2.
Primer sequences and product sizes for RT-PCR.
| Primer | Sequence | Product Size |
|---|---|---|
| ΔN Sense | GGAAAACAATGCCCAGACTC | |
| p63α Anti-sense | ATGATGAACAGCCCAACCTC | 1389 |
| p63β Anti-sense | CAGACTTGCCAGATCCTGA | 1376 |
| p63γ Anti-sense | GGGTACACTGATCGGTTTGG | 1168 |
| β-actin | ||
| Sense | GAGCGCAAGTACTCCGTGT | |
| Anti-sense | GCACGAAGGCTCATCATTCAAA | 548 |
Live/Dead Viability/Cytotoxicity Assay
2×104 cells/dish were plated onto collagen type 1 coated MatTek dishes (MatTek Corporation, Ashland, MA) and allowed to adhere overnight. Media was changed and cells were allowed to grow for 6 or 24 hours in media alone or media containing 150 μM CoCl2. Parallel non-treated samples were used as controls in all experiments. Prior to staining, media was removed and cells were washed twice in PBS. Viability was assessed using a Live/Dead Viability/Cytotoxicity Assay (Invitrogen, Carlsbad, CA) by staining with 2 μM Calcein-AM and 4 μM Ethidium-1 homodimer in PBS for 45 minutes at 37°C. Representative areas were imaged using a Leica SP2 laser scanning confocal microscope (Leica Instruments GmBH, Nussloch, Germany) with a 20x dry objective.
Statistics
Statistical analysis was performed using SigmaStat 3.1 (Systat Software, Inc., San Jose, CA). All data are expressed in mean ± standard deviation and representative of three or more combined experiments. Normality and equal variance assumption testing were performed using the Kolmogorov-Smirnov Test and the Levene median test. For comparisons between two groups, a t-test was used to determine which groups were significantly different. Statistical significance was set at p<0.05.
Results
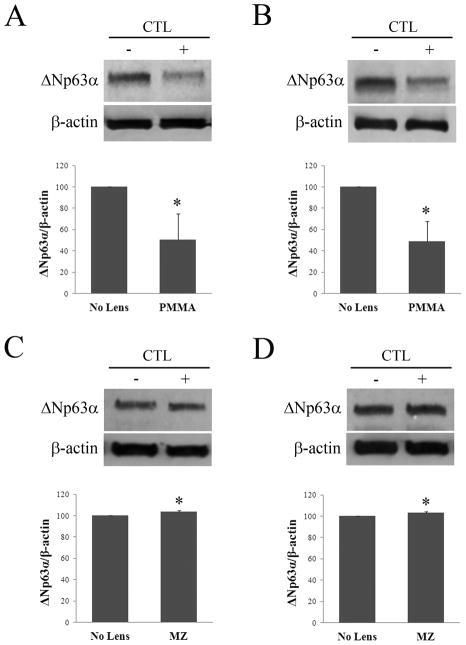
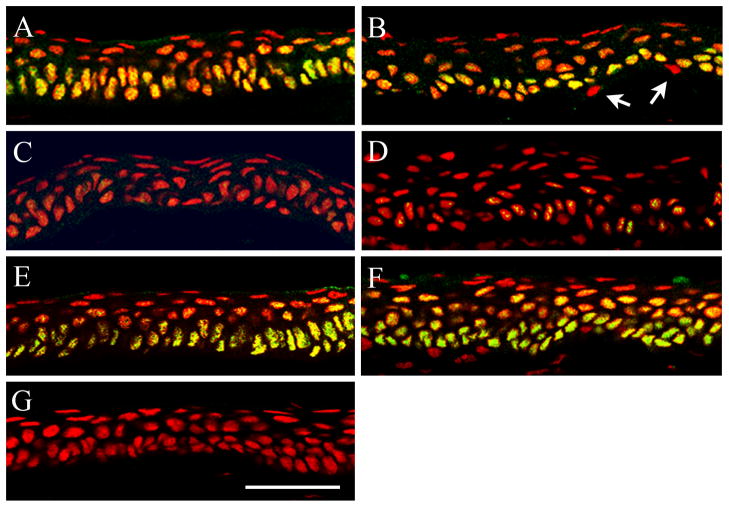
Hypoxic effects of low oxygen transmissible contact lenses on ΔNp63α expression in vivo were evaluated after fitting rabbits with either a PMMA (zero oxygen transmission) or a hyper-oxygen transmissible (Menicon Z) RGP contact lens in one eye for 24 hours of wear (Table 1). Compared to the non-lens wearing eye, western blotting for ΔNp63α demonstrated a decrease following PMMA lens wear in both the central (Figure 1A, p=0.025) and limbal epithelium (Figure 1B, p=0.009). In contrast, ΔNp63α expression was not reduced but appeared slightly increased after wear of the hyper-oxygen RGP lens (cornea, Figure 1C, p=0.005; limbus, Figure 1D, p=0.004). To correlate these changes with protein localization in vivo, immunofluorescent staining of ΔNp63 was performed after 24 hours of PMMA and hyper-oxygen transmissible lens wear (Figure 2). In the non-lens wearing rabbit cornea, ΔNp63 expression was strongest in nuclei throughout the entire basal epithelium, with weaker staining noted in epithelial nuclei within the wing cell layer, just above the basal cells (cornea, Figure 2A; limbus, Figure 2B). ΔNp63α expression was completely absent in all surface epithelial cells in the central cornea and limbus. Occasional ΔNp63 negative cells were also seen throughout the basal cell layer in the limbus (Figure 2B). Following 24 hours of PMMA lens wear, intensity of nuclear expression of ΔNp63 was greatly reduced and barely detectable throughout much of the epithelium (cornea, Figure 2C; limbus, Figure 2D). In contrast, 24 hours of lens wear with a hyper-oxygen transmissible lens material failed to alter nuclear expression of ΔNp63 compared to the non-lens wearing control (cornea, Figure 2C; limbus, Figure 2D).
Figure 1.
Western blot for ΔNp63α following 24 hours of contact lens wear. Proteins were resolved on a 7.5% SDS gel. (A–B) Wear of a non-oxygen transmissible PMMA lens significantly reduced ΔNp63α expression in the central corneal epithelium (A, p=0.025, t-test, n=3); and the limbal epithelium (B, p=0.009, t-test, n=3). (C–D) In contrast, ΔNp63α expression was not reduced following wear of a hyper oxygen transmissible lens (MZ) in the central corneal epithelium (C, p=0.005, t-test, n=3); or the limbal epithelium (D, p=0.004, t-test, n=3). β–actin was used as a loading control for all experiments. CTL: contact lens; (+) with lens; (−) no lens.
Figure 2.
Co-localization of ΔNp63 (green) and PI (red) to label epithelial nuclei (red) in the rabbit corneal epithelium following overnight contact lens wear. (A–B) In the non-lens wearing eye, ΔNp63 localized to the nuclei of all epithelial basal cells throughout the central corneal (A) and limbal (B) epithelium. Weaker expression was seen in epithelial nuclei in the wing cell layer. Expression was absent in all superficial cells. Occasional ΔNp63 negative cells were noted in the basal layer of the limbus (arrows). (C–D) 24 hours of PMMA lens wear decreased nuclear expression of ΔNp63 throughout the central corneal (C) and limbal (D) epithelium. (E–F) ΔNp63 expression and localization was unaltered after 24 hours of hyper-oxygen transmissible RGP lens wear compared to the control eye; central corneal (E) and limbal (F) epithelium. (G) Negative control, primary antibody omitted. Scale: 48 μm. Images representative of repeated experiments.
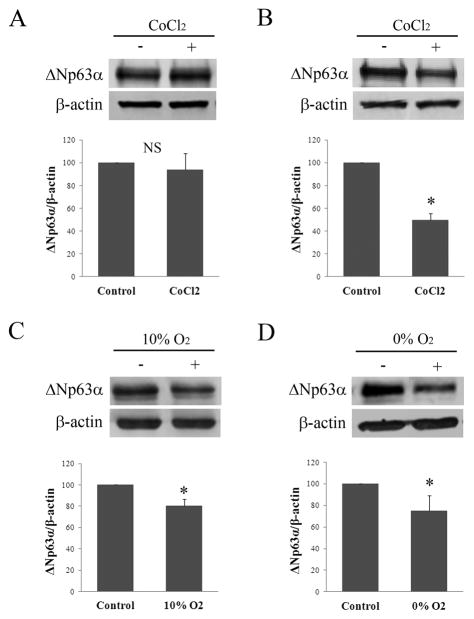
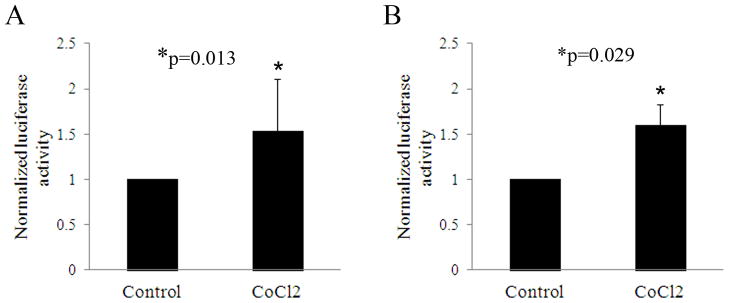
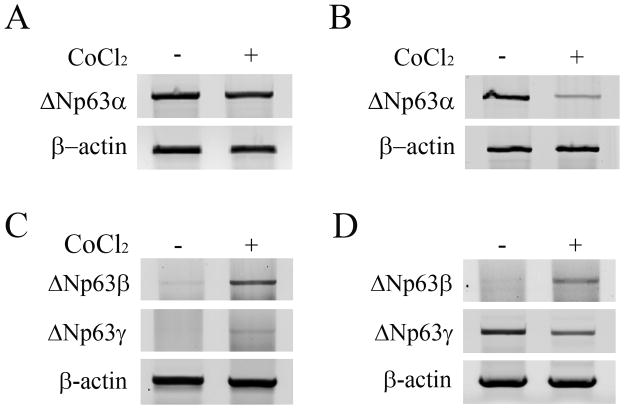
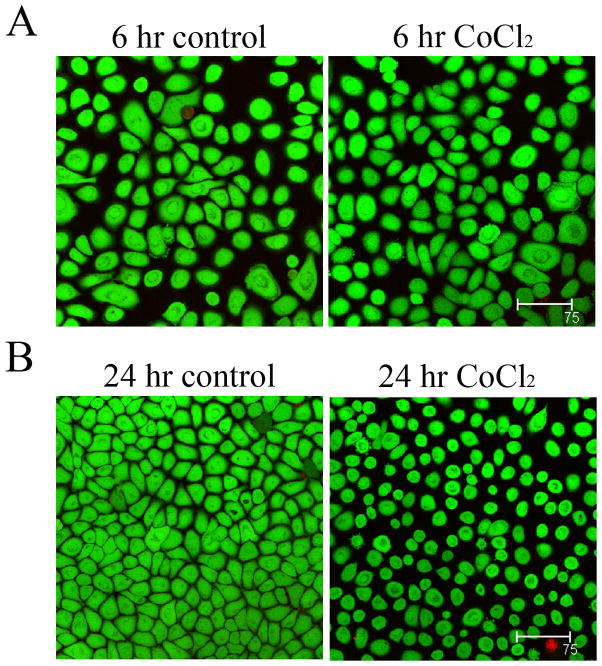
In vitro, 6 hours of CoCl2 treatment failed to alter expression compared to non-treated controls (Figure 3A, p=0.495). At 24 hours however, ΔNp63α expression was decreased compared to the non-treated control (Figure 3B, p<0.001). Similar to 24 hours of CoCl2 treatment, incubation in 10% hypoxia induced a slight reduction in ΔNp63α, which was greater following incubation in anoxia (Figure 3C, p=0.005 and p=0.029, 10% hypoxia and anoxia, respectively). The effect of CoCl2 on ΔNp63 transcriptional activity was then evaluated in cultured corneal epithelial cells after treatment for 6 and 24 hours. At both 6 and 24 hours of treatment, luciferase assays showed a significant upregulation in ΔNp63 activity (p=0.013 and p=0.029 at 6 and 24 hours, respectively, Figures 4A and B). In contrast, semi-quantitative RT-PCR for ΔNp63α showed no effect from CoCl2 treatment at 6 hours, but decreased ΔNp63α mRNA at 24 hours (Figure 5, A and B). ΔNp63γ was similarly reduced at 24 hours, whereas ΔNp63β increased at both time points (Figure 5, C and D). Live/dead assay demonstrated occasional non-viable cells in all test groups; however, no significant toxicity from CoCl2 treatment was seen (Figure 6). A change in cell morphology at 24 hours as cells was detected; cells were smaller and more rounded with signs of membrane blebbing (Figure 6B).
Figure 3.
Effects of hypoxia on ΔNp63α expression in hTCEpi cells in vitro. (A) There was no effect on ΔNp63α expression after 6 hours of treatment with CoCl2 compared to non-treated cells (p=0.495, t-test, n=3). (B) In contrast, a significant decrease in ΔNp63α was seen after 24 hours of treatment with CoCl2 (p<0.001, t-test, n=3). (C) A smaller, but significant reduction was seen in ΔNp63α following culture in 10% hypoxia for 24 hours (p=0.005, t-test, n=3); the decrease was more evident following 24 hour culture in 0% hypoxia (p=0.029, t-test, n=4).β–actin was used as a loading control for all experiments. (+) treatment group; (−) control.
Figure 4.
ΔNp63 transcriptional activity. (A&B) Luciferase activity after treatment with CoCl2 for the indicated time points compared to parallel control cultures. (A) Treatment with CoCl2 for 6 hours resulted in a significant increase in ΔNp63 activity compared to the non-treated control (p=0.013, t-test, n=4). (B) Treatment with CoCl2 for 24 hours also showed a corresponding increase in ΔNp63 activity compared to the non-treated control (p=0.029, t-test, n=3). Data was normalized to Renilla expression to control for variations in transfection efficiency. Due to the potential for variation in promoter activity under different culture conditions, data was then normalized to the pGL3 empty vector control. Non-CoCl2 treated controls were set at 1.
Figure 5.
Semi-quantitative RT PCR for ΔNp63 isoforms in hTCEpi cells. (A) There was no apparent difference in ΔNp63α mRNA levels after 6 hours of incubation in CoCl2. (B) After 24 hours of treatment, ΔNp63α mRNA was reduced compared to non-treated controls. (C) Treatment with CoCl2for 6 hours increased ΔNp63β, with little effect on ΔNp63γ. (D) After 24 hours, ΔNp63β mRNA levels remained elevated with a corresponding reduction in ΔNp63γ. Images representative of 3 repeated experiments. β-actin was used a housekeeping gene.
Figure 6.
Live/Dead assay for cell viability. hTCEpi cell viability was assessed using live/dead Calcein/ethidium staining after 6 and 24 hours of treatment with CoCl2. Viable cells in green, non-viable cells in red. (A) hTCEpi cells incubated with or without CoCl2 for 6 hours; (B) hTCEpi cells incubated with or without CoCl2 for 24 hours. Calcein staining confirmed the absence of significant toxicity resulting from CoCl2 treatment. Scale: 75 μm.
Discussion
The important new finding in this study is the identification of the relationship between ΔNp63α and hypoxic stress in the corneal epithelium in vivo. Specifically, the results demonstrate that ΔNp63α is down regulated in corneal epithelial cells when challenged under severe hypoxic stress. This occurs in vitro after prolonged culture (24 hours or more) and is not evident after short term hypoxic exposure of only 6 hours, a time period more reflective of normal eyelid closure during sleep. This finding is supported by in vivo animal modeling, whereby hypoxic stress induced by large diameter PMMA contact lens wear resulted in a decrease in ΔNp63α throughout the limbal and corneal epithelium. In direct contrast to PMMA lens wear which results in an equivalent oxygen percentage at the corneal surface of 0, wear of a hyper-oxygen transmissible rigid gas permeable lens with an equivalent oxygen percentage of 19 under open-eye conditions failed to demonstrate any detectable loss of ΔNp63α. Instead, wear of the hyper oxygen transmissible lens resulted in a small, but statistically significant increase in ΔNp63α, which is not likely clinically relevant due to the small magnitude of change.
The use of an alignment fit hyper-oxygen transmissible rigid lens furthers supports that the loss of ΔNp63α evident with PMMA lens wear was a direct consequence of lens-induced hypoxia and not only a result of the mechanical presence of the lens. Previous studies in the rabbit model have demonstrated that corneal epithelial surface damage induced by hypoxic lens wear is directly proportional to lens-oxygen transmissibility.31 At the basal cell layer, proliferation rate is similarly correlated with lens oxygen transmissibility; however, at similar Dk/t values, rigid lenses induce a greater inhibition on proliferation that hydrogel lenses, suggesting a potential for synergistic effects between hypoxia and mechanical influences.4, 11, 12 While both hypoxia and cobalt chloride treatment reduced ΔNp63α expression in vitro, further studies are necessary to investigate the differential effects of the lens in mediating hypoxia-induced changes in ΔNp63α in the corneal epithelium.
Previous studies have investigated the effects of hypoxia on limbal epithelial cells in vitro and have yielded conflicting results.32–34 In one study, culture in 2% oxygen had no effect on ΔNp63 expression and resulted in increased proliferation compared to normoxic controls while inhibiting differentiation as assessed by expression of involucrin and K3.32 In contrast, a more recent investigation cultured cells in various concentrations of oxygen and suggested that mild hypoxia (14%) enhanced culture and more severe levels of hypoxia (8% or less) resulted in a decrease in ΔNp63α mRNA that was associated with reduced proliferative capacity and enhanced differentiation.34 Importantly, the latter of these supports our current finding that hypoxia downregulates ΔNp63α in vitro through transcriptional mechanisms. Interestingly however, was the increase in ΔN promoter activity that was seen at 6 and 24 hours of treatment. This suggests that while message levels for the alpha isoform are reduced, there is a potential increase in other ΔNp63 isoforms and is supported by the increase in ΔNp63β mRNA detected by RT PCR under the current culture conditions. The significance of changes in different ΔNp63 isoforms in response to hypoxia requires further investigation.
The failure of hypoxia to alter ΔNp63α transcription or protein expression after only 6 hours would suggest that homeostatic mechanisms are resistant to short term hypoxia and that longer sustained periods are necessary to induce damage. This is supported by our in vitro live/dead data and is in agreement with earlier clinical studies in which short term hypoxic exposure of the eye to an anoxic environment failed to induce cellular damage illustrated by a subsequent bacterial binding response,14 as compared to 24 hour lens wear that induces an increase in bacterial binding that is directly proportional to lens oxygen transmissibility.16, 35
It is important to note however, that in this study we used the contralateral eye as a control, similar to what has been previously reported in the rabbit contact lens model. In these prior investigations, BrdU-labeling has demonstrated a sympathetic response between eyes, in which the perturbation to one eye can induce a smaller but similar corresponding effect in the contralateral eye.11 This finding has also been reported in human clinical studies on corneal epithelial swelling and in animal models of wound healing.36, 37 The potential relevance of a sympathetic response to the present study would suggest that a decrease in ΔNp63α levels in one eye may be accompanied by a corresponding, albeit lesser decrease in ΔNp63α levels in the contralateral eye; therefore the reduction detected in this study in response to lens-induced hypoxia may be underestimated.
Similar to early reports on the localization of ΔNp63 in the corneal epithelium,24 we noted the presence of occasional ΔNp63 negative cells in the basal layer of the limbus. In contrast to earlier studies however, the localization pattern of ΔNp63α within the undisturbed corneal epithelium extends well beyond the limbal compartment and was prominent throughout the basal cell layer of the central corneal epithelium. This is in agreement with our earlier findings which incorporated the use of a blocking peptide to demonstrate specificity,19 and is also consistent with the mRNA findings reported by others.26 While variations in staining protocols may contribute to the disparity reported in protein localization between groups, the findings in this study suggests that the origin of the donor tissue and the duration of eyelid closure prior to tissue collection, particularly if the human donor has been on a ventilator under closed-eye conditions for prolonged periods prior to death, may account in part, for this difference.
In summary, the decrease in ΔNp63α observed in the in vivo rabbit model is a direct result of prolonged hypoxia-induced transcriptional down regulation measured by both low gas culture and cobalt chloride and is associated with loss of cell viability. These findings suggest that the loss of ΔNp63α that occurs during prolonged hypoxia induced by contact lens wear or eyelid closure may contribute to corneal epithelial damage reported in animal studies. Further, the use of the well-established contact lens model to evaluate changes in protein expression in the central corneal epithelium supports the hypothesis that ΔNp63α mediates corneal epithelial homeostatic events outside of the limbal compartment.
Acknowledgments
Supported in Part by NIH Grant R01 EY018219 (DMR), Core Grant EY020799, OneSight Research Foundation, Dallas, Texas (DMR), and a Career Development Award (DMR) and an unrestricted grant from Research to Prevent Blindness, Inc., New York, New York.
Footnotes
CR: The authors have no conflicts of interest to disclose.
References
- 1.Thoft RA, Friend J. The X, Y, Z hypothesis of corneal epithelial maintenance. Invest Ophthalmol Vis Sci. 1983;24:1442–1443. [PubMed] [Google Scholar]
- 2.Cavanagh HD, Robertson DM, Petroll WM, et al. Castroviejo Lecture 2009: 40 years in search of the perfect contact lens. Cornea. 2010;19:1075–1085. doi: 10.1097/ICO.0b013e3181d103bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cavanagh HD. The effects of low- and hyper-Dk contact lenses on corneal epithelial homeostasis. Ophthalm Clin N Am. 2003;16:311–325. doi: 10.1016/s0896-1549(03)00044-0. [DOI] [PubMed] [Google Scholar]
- 4.Ladage PM, Yamamoto K, Li L, et al. Corneal epithelial homeostasis following daily and overnight contact lens wear. Contact Lens & Ant Eye. 2002;25:11–21. doi: 10.1016/s1367-0484(01)00003-0. [DOI] [PubMed] [Google Scholar]
- 5.Robertson DM, Petroll WM, Jester JV, et al. Current concepts: contact lens related Pseudomonas keratitis. Cont Lens Ant Eye. 2007;30:94–107. doi: 10.1016/j.clae.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Cavanagh HD, Ladage PM, Li SL, et al. Effects of daily and overnight wear of a novel hyper oxygen-transmissible soft contact lens on bacterial binding and corneal epithelium: a 13-month clinical trial. Ophthalmology. 2002;109:1957–1969. doi: 10.1016/s0161-6420(02)01278-2. [DOI] [PubMed] [Google Scholar]
- 7.Ladage PM, Yamamoto K, Ren DH, et al. Effects of rigid and soft contact lens daily wear on corneal epithelium, tear lactate dehydrogenase, and bacterial binding to exfoliated epithelial cells. Ophthalmology. 2001;108:1279–1288. doi: 10.1016/s0161-6420(01)00639-x. [DOI] [PubMed] [Google Scholar]
- 8.Ren DH, Petroll WM, Jester JV, et al. The relationship between contact lens oxygen permeability and binding of Pseudomonas aeruginosa to human corneal epithelial cells after overnight and extended wear [published erratum appears in CLAO J 1999 25:175] CLAO J. 1999b;25:80–100. [PubMed] [Google Scholar]
- 9.Ren DH, Petroll WM, Jester JV, et al. The effect of rigid gas permeable contact lens wear on proliferation of rabbit corneal and conjunctival epithelial cells. CLAO J. 1999a;25:136–141. [PubMed] [Google Scholar]
- 10.Li L, Ren DH, Ladage PM, et al. Annexin V Binding to Rabbit Corneal Eptihelial Cells Following Overnight Contact Lens Wear or Eyelid Closure. The CLAO J. 2002;28:48–54. [PubMed] [Google Scholar]
- 11.Ladage PM, Ren DH, Petroll WM, et al. Effects of Eyelid Closure and Disposable and Silicone Hydrogel Extended Contact Lens Wear on Rabbit Corneal Epithelial Proliferation. Invest Ophthalmol Vis Sci. 2003;44:1843–1849. doi: 10.1167/iovs.02-0897. [DOI] [PubMed] [Google Scholar]
- 12.Ladage PM, Yamamoto K, Ren DH, et al. Proliferation Rate of Rabbit Corneal Epithelium during Overnight Rigid Contact Lens Wear. Invest Ophthalmol Vis Sci. 2001;42:2804–2812. [PubMed] [Google Scholar]
- 13.Ladage PM, Jester JV, Petroll WM, et al. Vertical Movement of Epithelial Basal Cells toward the Corneal Surface during Use of Extended-Wear Contact Lenses. Invest Ophthalmol Vis Sci. 2003;44:1056–1063. doi: 10.1167/iovs.02-0725. [DOI] [PubMed] [Google Scholar]
- 14.Ren DH, Petroll WM, Jester JV, et al. Short-term hypoxia downregulates epithelial cell desquamation in vivo, but does not increase Pseudomonas aeruginosa adherence to exfoliated human corneal epithelial cells. CLAO J. 1999;25:73–79. [PubMed] [Google Scholar]
- 15.Ladage PM, Yamamoto K, Li L, et al. Effects of O2-transmissibility on corneal epithelium after daily and extended contact lens wear in rabbit and man. Adv Exp Med Biol. 2002;506:885–893. doi: 10.1007/978-1-4615-0717-8_125. [DOI] [PubMed] [Google Scholar]
- 16.Imayasu M, Petroll WM, Jester JV, et al. The relationship between contact lens oxygen transmissibility and binding of Pseudomonas aeruginosa to the cornea after overnight wear. Ophthalmology. 1994;101:371–388. doi: 10.1016/s0161-6420(94)31326-1. [DOI] [PubMed] [Google Scholar]
- 17.Ichijima H, Ohashi J-I, Cavanagh HD. Effect of contact-lens-induced hypoxia on lactate dehyrogenase activity and isozyme in rabbit cornea. Cornea. 1992;11:108–113. doi: 10.1097/00003226-199203000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto K, Ladage PM, Ren DH, et al. Effect of Eyelid Closure and Overnight Contact Lens Wear on Viability of Surface Epithelial Cells in Rabbit Cornea. Cornea. 2002;21:85–90. doi: 10.1097/00003226-200201000-00018. [DOI] [PubMed] [Google Scholar]
- 19.Robertson DM, Ho SI, Cavanagh HD. Characterization of DeltaNp63 isoforms in normal cornea and telomerase-immortalized human corneal epithelial cells. Exp Eye Res. 2008;86:576–585. doi: 10.1016/j.exer.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koster MI, Kim S, Mills AA, et al. p63 is the molecular switch for initiation of an epithelial stratification program. Genes Dev. 2004;18:126–131. doi: 10.1101/gad.1165104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mills AA, Zheng BH, Wang XJ, et al. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999;398:708–713. doi: 10.1038/19531. [DOI] [PubMed] [Google Scholar]
- 22.Van Bokhoven H, McKeon F. Mutations in the p53 homolog p63: allele-specific developmental syndromes in humans. Trends in Mol Med. 2002;8:133–139. doi: 10.1016/s1471-4914(01)02260-2. [DOI] [PubMed] [Google Scholar]
- 23.Yang A, Kaghad M, Wang Y, et al. p63, a p53 Homolog at 3q27–29, Encodes Multiple Products with Transactivating, Death-Inducing, and Dominant-Negative Activities. Mol Cell Biol. 1998;2:305–316. doi: 10.1016/s1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- 24.Pellegrini G, Dellambra E, Golisano O, et al. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci USA. 2001;98:3156–3161. doi: 10.1073/pnas.061032098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Iorio E, Barbaro V, Ruzza A, et al. Isoforms of DeltaNp63 and the migration of ocular limbal cells in human corneal regeneration. Proc Natl Acad Sci USA. 2005;102:9523–9528. doi: 10.1073/pnas.0503437102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawasaki S, Tanioka H, Yamasaki K, et al. Expression and tissue distribution of p63 isoforms in human ocular surface epithelia. Exp Eye Res. 2006;82:293–299. doi: 10.1016/j.exer.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Robertson DM, Li L, Fisher S, et al. Characterization of Growth and Differentiation in a Telomerase-Immortalized Human Corneal Epithelial Cell Line. Invest Ophthalmol Vis Sci. 2005;46:470–478. doi: 10.1167/iovs.04-0528. [DOI] [PubMed] [Google Scholar]
- 28.Ouiddir A, Planes C, Fernandes I, et al. Hypoxia upregulates activity and expression of the glucose transporter GLUT1 in alveolar epithelial cells. Am J Respir Cell Mol Biol. 1999;21:710–718. doi: 10.1165/ajrcmb.21.6.3751. [DOI] [PubMed] [Google Scholar]
- 29.Vengellur A, LaPres JJ. The role of hypoxia inducible factor 1α in cobalt chloride induced cell death in mouse embryonic fibroblasts. Toxicol Sci. 2004;82:638–646. doi: 10.1093/toxsci/kfh278. [DOI] [PubMed] [Google Scholar]
- 30.Ji Z, Yang G, Shahzidi S, et al. Induction of hypoxia-inducible factor-1α overexpression by cobalt chloride enhances cellular resistance to photodynamic therapy. Cancer Lett. 2006;244:182–189. doi: 10.1016/j.canlet.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 31.Imayasu M, Petroll WM, Jester JV, et al. The relation between contact lens oxygen transmissibility and binding of Pseudomonas aeruginosa to the cornea after overnight wear. Ophthalmology. 1994;101:371–388. doi: 10.1016/s0161-6420(94)31326-1. [DOI] [PubMed] [Google Scholar]
- 32.Miyashita H, Higa K, Kato N, et al. Hypoxia enhances the expansion of human limbal epithelial progenitor cells in vitro. Invest Ophthalmol Vis Sci. 2007;48:3586–3593. doi: 10.1167/iovs.07-0077. [DOI] [PubMed] [Google Scholar]
- 33.Li C, Yin T, Dong N, et al. Oxygen tension affects terminal differentiation of corneal limbal epithelial cells. J Cell Physiol. 2010 doi: 10.1002/jcp.22591. [DOI] [PubMed] [Google Scholar]
- 34.O’Callaghan AR, Daniels JT, Mason C. Effect of sub-atmospheric oxygen on the culture of rabbit limbal epithelial cells. Curr Eye Res. 2011;36:691–698. doi: 10.3109/02713683.2011.556302. [DOI] [PubMed] [Google Scholar]
- 35.Ren H, Petroll WM, Jester JV, et al. Adherence of Pseudomoonas aeruginosa to shed rabbit corneal epithelial cells after overnight wear of contact lenses. CLAO J. 1997;23:63–68. [PubMed] [Google Scholar]
- 36.Fonn D, du Toit R, Simpson TL, et al. Sympathetic swelling response of the control eye to soft lenses in the other eye. Invest Ophthalmol Vis Sci. 1999;40:3116–3121. [PubMed] [Google Scholar]
- 37.Jones MA, Marfurt CF. Sympathetic stimulation of corneal epithelial proliferation in wounded and nonwounded rat eyes. Invest Ophthalmol Vis Sci. 1996;37:2535–2547. [PubMed] [Google Scholar]