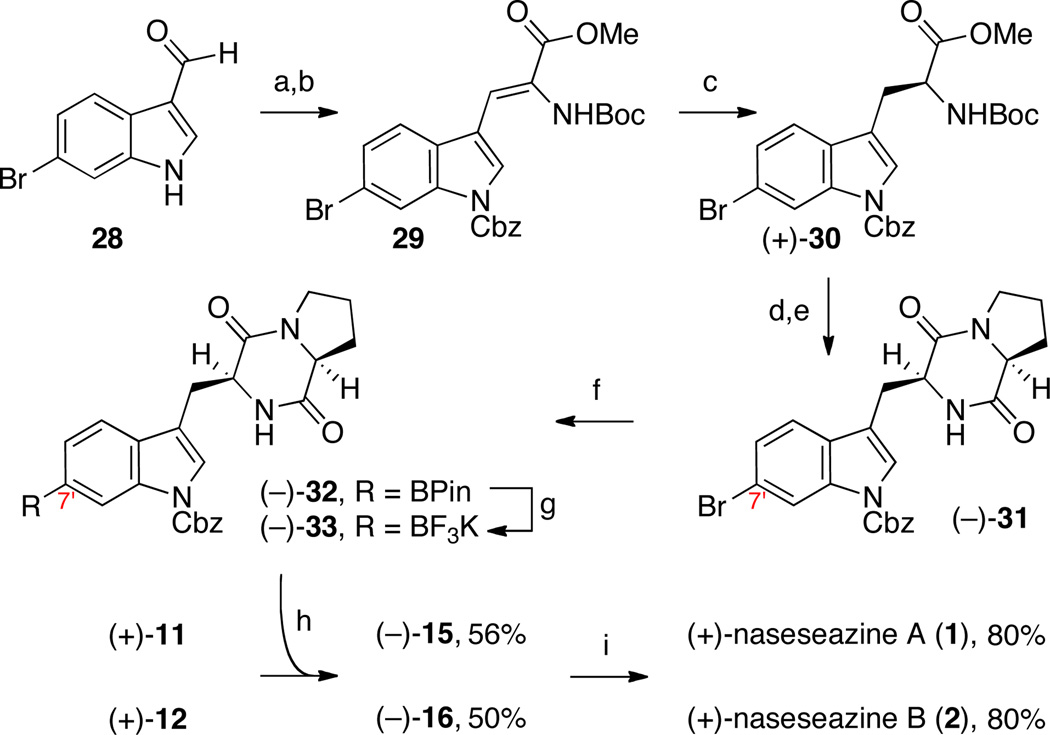
Scheme 3.
Concise and directed synthesis of (+)-naseseazines A (1) and B (2).a
a Conditions: (a) CbzCl, Et3N, DMAP, CH2Cl2, 100%. (b) Boc-α-phosphonoglycine trimethylester, DBU, CH2Cl2, 97%. (c) H2 (80 psi), (S,S)-Et-DUPHOS-Rh (1.8 mol%), CH2Cl2, MeOH, 97%, >99% ee. (d) TFA, CH2Cl2; EDC•HCl, HOBt, Et3N, Boc-l-Pro. (e) TFA, CH2Cl2; NH4OH, MeOH, 75% (2-steps). (f) 2-aminobiphenyl(XPhos)PdCl (5 mol%), XPhos (15 mol%), (BPin)2, K3PO4, DMSO, 60 °C, 65%. (g) KHF2 (aq.), MeOH, 88%. (h) AgSbF6, 18-crown-6, EtNO2, 23 °C. (i) H2, Pd/C, AcOH, 23 °C.