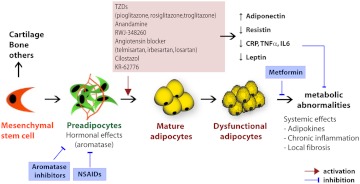
Fig. 4.
Pharmacological options for modulating adipose tissue-associated hormonal and metabolic effects. Stromal preadipocytes of adipose tissue, which are derived from mesenchymal stem cells or EMT, are a major source of aromatase activity. To modulate hormonal effects of adipose tissues, two different approaches have been entertained: 1) induction of preadipocyte differentiation to healthy adipocytes; and 2) inhibition of aromatase activity. A variety of pharmacological agents are available to induce preadipocyte differentiation. Most agents that induce preadipocyte differentiation do so through PPARγ-dependent mechanisms. This results in increased adiponectin levels, reduced inflammation, and improved insulin sensitivity. Steroidal (type I) and nonsteroidal (type II) aromatase inhibitors and NSAID have been widely used to inhibit aromatase activity. From a metabolism perspective, systemic metabolic abnormalities due to dysfunctional adipocytes in obesity are key risk factors of tumor progression. Agents that normalize metabolic abnormalities, such as insulin resistance, chronic inflammation, and adipokine dysregulation, are potentially useful to treat obesity-related cancers. In this regard, PPARγ agonists and metformin, both widely used agents in the diabetes clinic, are under investigation. CRP, C-reactive protein.