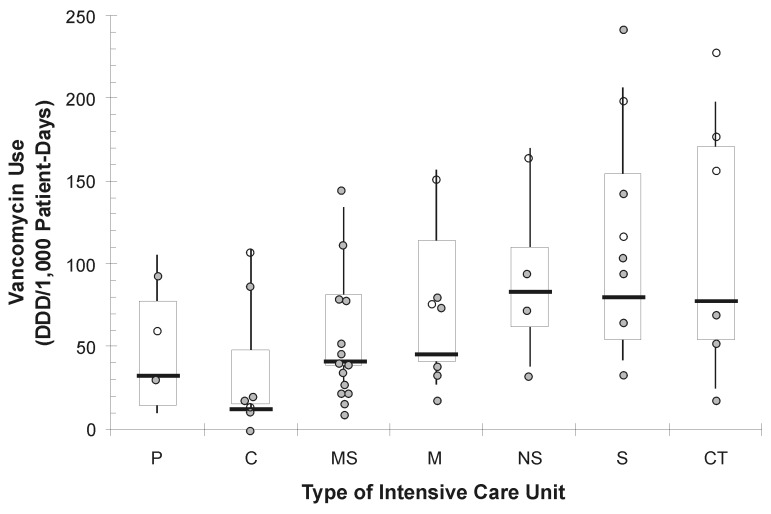
Figure 1.
Boxplot of benchmark data of vancomycin use at all Phase 2 Project Intensive Care Antimicrobial Resistance Epidemiology (ICARE) hospitals (n=113 intensive-care units [ICUs]) in October 1997, by type of ICU (18). ICU types include pediatric (P), coronary (C), combined medical-surgical (MS), neurosurgical (NS), surgical (S), and cardiothoracic (CT). For each type of ICU, boxes represent rates of vancomycin use at the 25th–75th percentiles (interquartile range), and ends of vertical lines represent values at the 10th–90th percentiles. Horizontal lines represent median values in each ICU type. Additionally, plotted circles represent the rate of vancomycin use in the pre-intervention period (1996–1997) in the 50 ICUs participating in the intervention study, and open circles represent the 10 ICUs reporting a prescriber practice change identified in the specific unit (i.e., ICU-specific practice change) (1 burn ICU not shown). DDD, defined daily doses.