Table 1.
Dioxygenation of 1,3-dienes via diboration/oxidation.[a]
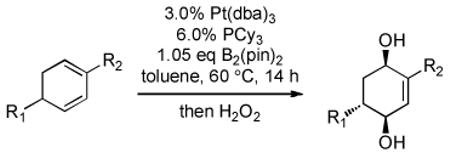
| entry | substrate | product | yield [%][b] | dr[c] |
|---|---|---|---|---|
| 1 | 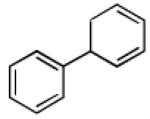 |
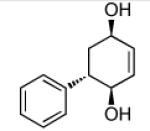 |
86 | >20:1 |
| 2 | 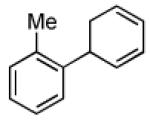 |
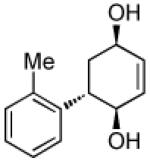 |
71 | >20:1 |
| 3 | 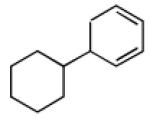 |
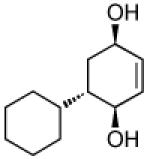 |
93 | >20:1 |
| 4 | 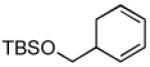 |
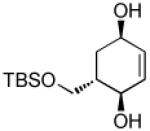 |
69 | >20:1 |
| 5[d] | 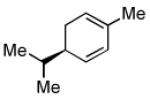 |
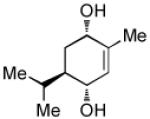 |
87 | >20:1 |
Reactions were conducted with exclusion of moisture and under a nitrogen atmosphere.
The yield represents the isolated yield of purified material and is an average of at least two experiments.
Diastereoselectivity was determined by 400 MHz 1H NMR analysis; (for >20:1, the minor diastereomer was not detected).
To achieve optimal yield with this substrate, the Pt(dba)3 and PCy3 were pre-mixed at rt for 1 h.
