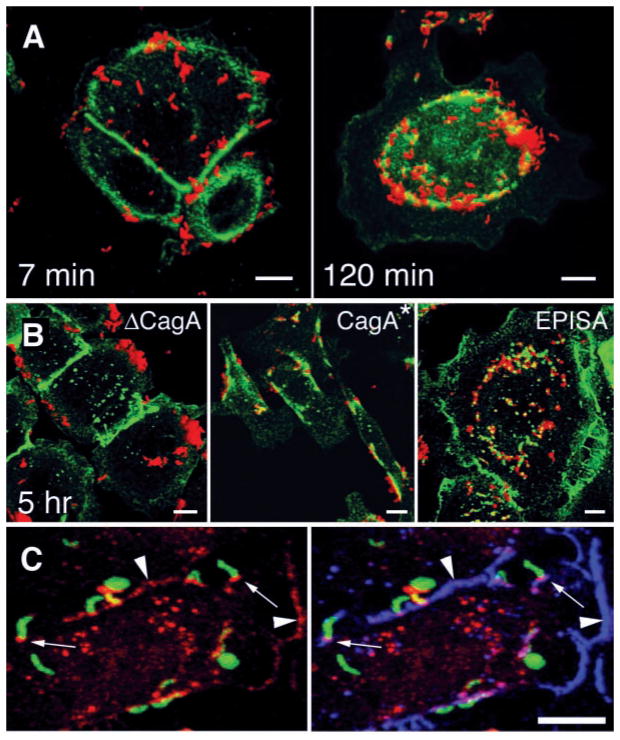
Fig. 2.
CagA is required for colocalization of H. pylori and the tight-junction protein ZO-1. [(A) and (B)] Confocal immunofluorescence 3D reconstructions of AGS cells infected with wild-type H. pylori and isogenic mutants, and stained with antibodies to H. pylori (red) and ZO-1 (green). (A) AGS cells were infected for 5 min with H. pylori, washed, and incubated for 7 or 120 min before fixation and staining. (B) AGS cells were incubated for 5 hours with an isogenic mutant lacking the cagA gene (ΔCagA), with wild-type reconstituted strain G27 CagA*, and with a mutant expressing a form of CagA that cannot be phosphorylated in tyrosine residues (EPISA). (C) Confocal immunofluorescence 3D reconstructions of AGS cells infected for 2 hours with H. pylori and modified to express GFP (green), and stained with antibodies to CagA (red) and ZO-1 (blue). Yellow represents areas of GFP and CagA colocalization, which are especially intense in some bacteria that became permeabilized to the antibodies during the staining. Colocalization of CagA and ZO-1 is present at sites of bacterial attachment (small arrows) and at cell-cell contact sites (arrowheads). Scale bar, 5 μm.