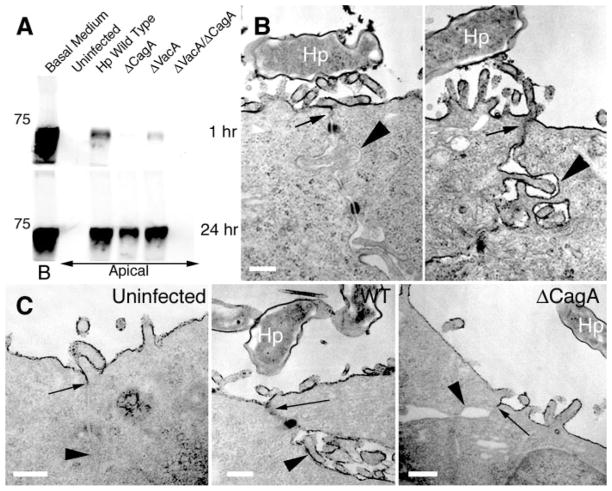
Fig. 3.
Infection of MDCK monolayers with H. pylori causes disruption of tight-junction barrier function and prevents normal junction formation. (A) Diffusion of biotinylated albumin across infected MDCK monolayers. Confluent monolayers on Transwell filters were infected with H. pylori or isogenic mutants in the apical chamber. At day 10 after infection, media in the basolateral chambers were marked with biotinylated albumin (Basal Medium, B) and samples were collected 1 hour and 24 hours later from the apical chambers. After separation by SDS–polyacrylamide gel electrophoresis (SDS-PAGE), the amount of biotinylated albumin in the apical chambers was visualized with a streptavidin probe. A molecular weight marker of 75 kD is indicated. (B) Transmission electron micrographs of two junctional areas in an MDCK cell monolayer infected with H. pylori (Hp) for 3 days, and fixed in the presence of the electron-dense dye ruthenium red. (C) MDCK cells infected before junction formation with wild-type (WT) or ΔCagA H. pylori. After 24 hours of infection, formation of functional tight junctions was determined by ruthenium red exclusion from the basal-lateral space. Arrows point to the location of tight junctions. Arrowheads show comparative ruthenium red staining of basal-lateral membranes. Scale bar, 0.25 μm.