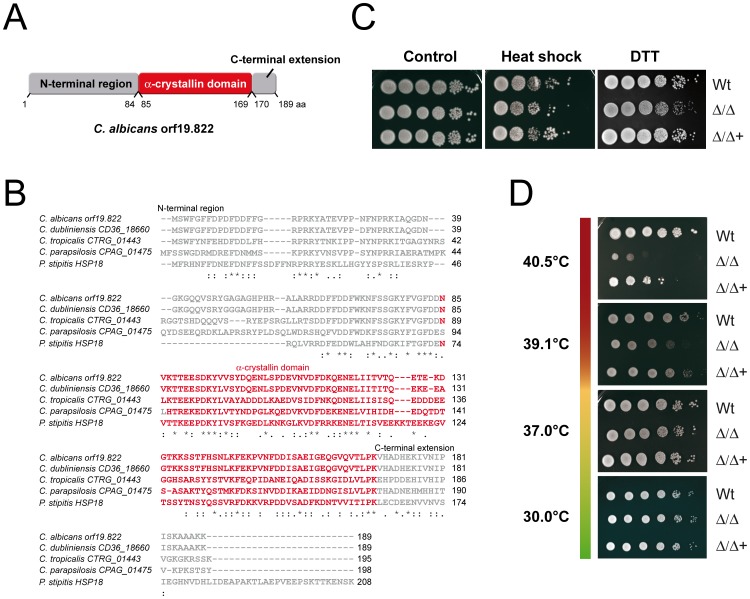
Figure 1. C. albicans orf19.822 encodes a predicted sHsp required for adaptation to long-term thermal stress.
(A) Structural organization of orf19.822 with a conserved central α-crystallin domain (red) flanked by variable N- and C-terminal domains (grey), based on results from http://www.expasy.ch/prosite/database. Numbers below the structural elements represent amino acid position. (B) Alignment of the orf19.822 protein sequence with orthologues from other organisms (generated with ClustalW2). The conserved α-crystallin-domain sequence is shown in red characters. Identical residues are marked with (*), residues with the same size and hydropathy are marked by (:), residues with the same size or hydropathy are marked by (.). (C) Short-term heat shock and endoplasmic reticulum (ER)-stress. Cells of YPD-overnight cultures of the wild type (Wt), hsp21Δ/Δ mutant (Δ/Δ) and hsp21Δ/Δ::HSP21 complemented mutant (Δ/Δ+) were serially diluted from 106 to 101 cells (left to right), either exposed to heat shock (50°C, 15 min) or not (control), plated on YPD and incubated for 2 days at 37°C. ER-stress was induced by growing the cells on YPD agar plates supplemented with 30 mM dithiothreitol (DTT). (D) Growth of the Wt, hsp21Δ/Δ mutant (Δ/Δ) and hsp21Δ/Δ::HSP21 complemented mutant (Δ/Δ+) on solid SD minimal medium at temperatures ranging from 30°C to 40.5°C.