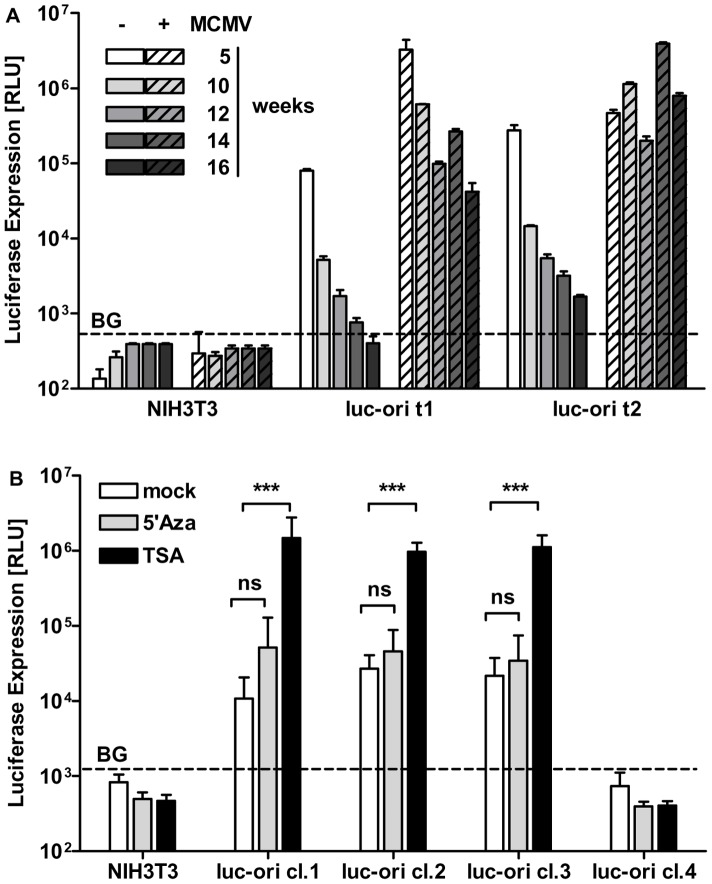
Figure 1. Infection with MCMV reactivates silenced replicon vector encoded reporter gene expression.
(A) In two stable NIH3T3 cell pools transfected with pEpibo-luc-ori (luc-ori t1, luc-ori t2) expression of FL was measured in absence of infection (plain bars, - MCMV) at the indicated weeks after transfection. Reporter gene expression is lost in uninfected cells over time. At the same time points the pools were infected with MCMV at an MOI of 0.5 (hatched bars, +MCMV). 24 h p.i. of both luc-ori t1 and t2 with high expression of FL was induced. NIH3T3 fibroblasts served as a control to determine background signal (BG) of the luciferin substrate. (B) Vector pEpibo-luc-ori was inactivated by histone deacetylation. Four cell clones (cl. 1–4) derived from subcloning of luc-ori t1 were subjected to treatment with 25 µM 5′ aza-cytidine (5′Aza, gray bars), an inhibitor of CpG-methylation, 330 nM Trichostatin A (TSA, black bars) for 36 h or left untreated (mock, open bars). FL expression was analyzed in comparison to parental NIH3T3 cells. FL expression was significantly enhanced by de-condensing histone packaging through TSA treatment (*** p<0.001, ns p>0.05, Two-Way-Anova, depicted is mean+SD). RLU (relative light units), p.i. post infection, weeks = weeks post transfection of pEpibo-luc-ori.