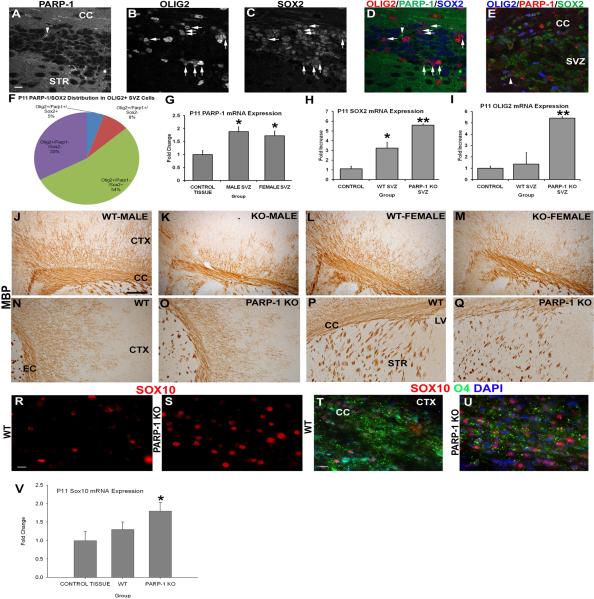
Figure 6. PARP-1 deletion upregulates expression of Sox2 and Sox10 and enhances the OPC population to compensate for the deficiency in myelination.
(A–E) Immunofluorescence labeling was performed on WT mice to identify Sox2, Olig2 and PARP-1 positive cells. PARP-1 is expressed at low levels throughout the brain, making it difficult to identify positive cells. In WT mice, very few SVZ cells expressed PARP-1 above baseline levels (A). These cells rarely expressed Olig2 (B) or Sox2 (C). Numerous Sox2+ cells (blue in D) are present in the SVZ (C) and many co-localize with Olig2 (red; arrows in D) but not PARP-1 (green in D). As a positive control for the PARP-1 antibody, the same immunofluorescence staining was performed on a SVZ section from a mouse exposed to hypoxia-ischemia, and upregulated PARP-1 expression is seen in the SVZ, as shown by an Olig2+/Sox2+/PARP-1+ cell (arrowhead in E). Close examination of the Olig2+ population in the SVZ of WT mice using confocal microscopy revealed that 54% of Olig2+ cells also express Sox2 but not PARP-1 while 33% of Olig2+ cells do not express either marker (F). On average, 8% of Olig2+ cells expressed PARP-1 in the SVZ while only 5% expressed all 3 markers (F). We examined mRNA expression in the SVZ to determine if PARP-1 was elevated in the WT mice. PARP-1 mRNA expression significantly increase in the SVZ of WT male and female mice compared to the nonneurogenic control tissue (G, *p<0.05). Sox2 mRNA expression increased in the SVZ of PARP-1 KO mice nearly 6-fold compared to the non-neurogenic control region and was significantly elevated compared to WT mice (H, **p<0.01). Olig2 mRNA expression was also increased nearly 6-fold in the SVZ of PARP-1 KO mice compared to the non-neurogenic control tissue (I). Olig2 mRNA expression was significantly elevated in the SVZ of PARP-1 knockout mice compared to the SVZ of WT mice (I, **p<0.01). (J–Q) Due to differences in oligodendrocyte progenitor numbers, we examined whether mature oligodendrocytes were affected in the PARP-1 KO mice. We performed immunohistochemistry with an antibody to myelin basic protein (MBP) to identify mature, myelinating oligodendrocytes. Examination of the corpus callosum overlying the SVZ revealed a decrease in the thickness of this area in PARP-1 KO mice (K, M) compared with WT mice (J, L). In addition to the thinner band of MBP-positive cells within the corpus callosum, fewer MBP+ cells appear to branch dorsally from the corpus callosum into the cortex and their location appears to be more restricted as well in PARP-1 KO mice (K, M) compared to WT mice (J, L). Examination of the external capsule revealed a similar reduction in MBP expression in PARP-1 KO mice (O) compared to WT mice (N), with KO mice displaying less dense MBP+ expression in the cortical region located lateral to the external capsule. Differences in MBP expression were less obvious in the striatum of PARP-1 KO mice (Q) compared with WT mice (P) but may be prevalent in this region as well. (R–U) We performed immunofluorescence to identify Sox10-positive and O4-positive oligodendroglial cells in the corpus callosum. We found enhanced Sox10 expression in the corpus callosum of PARP-1 KO mice (S) compared with WT mice (R). In addition, Sox10 mRNA expression measured by qPCR significantly increased in the PARP-1 KO as compared to WT mice (V, *p<0.05). Using O4 immunofluorescence staining, we noted decreased myelination in the corpus callosum of PARP-1 KO mice (U) compared with their WT counterparts (T). All data are shown with SEM. Scale bars: 10 μm in A (for A–E); 250 μm in J for J–P; 25 μm in R for R–S; 25 μm in T for T–U; CC: corpus callosum; STR: striatum; SVZ: subventricular zone; LV: lateral ventricle; CTX: cortex.