Abstract
Objectives
Metalworking fluids (MWFs) have been associated with cancer of several sites, but the risks have been primarily examined in men or in studies that adjusted for gender in analyses. To evaluate whether risks were similar in women, we report cancer mortality risk among 4,825 female autoworkers within the united autoworkers–general motors autoworkers cohort.
Methods
Standardized mortality rates (SMRs) were calculated based on Michigan death rates (1980–2004). Internal comparisons (1941–2004) were examined using Cox regression for straight, soluble, and synthetic MWFs, and their corresponding oil- and water-based fractions.
Results
MWF exposure levels in the female cohort were generally less than two-third the MWF levels in the male cohort. Female autoworkers had an excess of cancer from all sites (SMR, 1.10; 95 % confidence interval (CI), 0.98–1.22) and lung cancer (SMR, 2.08; 95 % CI, 1.71–2.52). Colon cancer risk increased with straight (mineral oil) MWF exposure (exposure>median; hazard ratio = 3.1; 95 % CI, 1.2–8.0). A protective effect was observed for ovarian cancer with the soluble MWFs and water-based MWF metrics. Although bladder, rectal, and laryngeal cancers and malignant melanoma have been associated with straight MWF exposure and pancreatic cancer with synthetic MWF in men, there were too few deaths in this female subcohort to examine exposure-response relations for these sites. Results were null for lung and breast cancer.
Conclusions
Our findings support an association between colon cancer and straight MWFs, but we found limited evidence of risk for other tumor sites at the lower exposure levels experienced by the female autoworkers.
Keywords: Metalworking fluids, Cancer mortality, Colon cancer, Women's health, Cohort studies
Introduction
Exposure to metalworking fluids (MWFs) has been associated with increased cancer risk for several cancer sites, including cancers of the bladder, larynx, lung, prostate, pancreas, rectum, and skin [1–16]. The evidence has come primarily from studies of males or in studies of cohorts that were predominately male where the analyses were adjusted for gender. While we assume risks identified in men also pertain to women, women may respond differently than men to the same occupational exposures because of metabolic, genetic, physiologic, or other differences [17–22]. In addition, the risk of female breast cancer and cancers of the female reproductive system cannot be evaluated in studies of men.
We report the risk of cancer mortality for a cohort of female autoworkers. These women are a subset from the united autoworkers-general motors (UAW-GM) cohort, which is the largest enumerated cohort exposed almost exclusively to MWFs. Although this cohort included over 4,800 women, there were too few cancer deaths in the 1985 and 1995 mortality follow-ups of this cohort to report the cancer risk for women separately [14, 15], with the exception of a case-control study of incident and mortal breast cancer [10]. As a result, previous mortality studies of this male-dominated cohort were based on pooled analyses of men and women, and no previous analysis examined the risk of female reproductive cancers. Endocrine disruption, a risk factor for reproductive cancers, has been hypothesized to occur with exposure to chlorinated hydrocarbons [23, 24], a component of oil-based MWFs. In addition, gender differences have been reported for some cancers, including lung cancer [25]. For instance, never smokers who develop lung cancer are more likely to be women than men [26]. These differences between men and women in lung cancer risk and disease histology suggest that estrogen may also be a risk factor for lung cancer and there is some biological evidence to support this hypothesis [27, 28].
In this paper, we report the external and internal comparisons of the risk of cancer mortality among female autoworkers with MWF exposure. The risk is examined in relation to quantitative estimates for the three broad classes of MWFs (straight, soluble, and synthetic), as well as for the fluids' oil-based (straight + soluble) and water-based (soluble + synthetic) fractions.
Methods
Cohort description
The female autoworkers mortality cohort was restricted to women hired on or after 1 January 1938 and before 1 January 1982 at one of the three Michigan UAW–GM plants (n = 4,825) to obtain a cohort hired during the follow-up period (incident hires) to reduce left truncation bias [29]. Left truncation bias can occur when subjects at risk prior to baseline are not all observable at the start of follow-up and can lead to incorrect conclusions about whether an association exists. This will occur in occupational cohort studies if subjects hired before the start of follow-up are included in analyses. Mortality follow-up was previously obtained for the period 1 January 1941 through 31 December 1994 [14, 15] and extended through 31 December 2004 with linkage to the National Death Index. Each subject's work history was obtained from company records. In internal comparisons, we excluded women with greater than 50 % missing work history information (n = 38). For subjects with <50 % missing work history information, we averaged the exposure levels of the jobs reported directly before and after the missing record (1.1 % of person-years). We conducted this study in accordance with a protocol approved by the office for the protection of human subjects at the University of California at Berkeley.
Exposure assessment
Quantitative exposure levels for straight, soluble, and synthetic MWF were previously estimated for each study year and linked to the subjects' work histories [14, 30, 31]. Straight, soluble, and synthetic MWFs have overlapping constituents, so we also calculated each subject's exposure to oil-based MWFs and water-based MWFs to account for the common constituents. Soluble MWF contributes to both the oil- and water-based fractions. We weighted the contribution of soluble MWF in each fraction to account for differences in potency between MWF types, using weights that were previously found to maximize the Wald statistic (slope parameter/standard error) in exposure–response relations of cancer incidence in the UAW–GM cohort [3]. Oil-based MWF exposure was calculated as the sum of straight MWF and soluble MWF, weighting the contribution of soluble MWF by 30 % (straight MWF + 0.3 * soluble MWF). Similarly, water-based MWF exposure was calculated as the sum of synthetic MWF and soluble MWF, weighting the contribution of soluble MWF by 20 % (synthetic MWF + 0.2 * soluble MWF). For each MWF metric, cumulative exposure was calculated for each subject using a lag of 10 years to account for the latency period before cancer diagnosis.
Statistical analyses
Standardized mortality rates (SMRs) were calculated using Michigan-specific mortality data (http://wonder.cdc.gov/mortsql.html; Accessed: June 20, 2011) for the period 1980 through 2004 (n = 4,553). Risk of cervical cancer in this cohort has been reported separately [32] and thus is not included in this report.
We evaluated exposure-response relations for cancer sites with 10 or more cases in internal comparisons using Cox regression models with age as the time metric and time-varying exposures (Stata/SE, version 11.2; StataCorp LP, College Station, Texas). The follow-up period for this internal analysis was 1941–2004. Each subject was followed from three years after date of hire to death or study end. Each model included covariates for race and calendar year (categorical, in 10-year intervals). In separate models for each outcome and exposure metric, we treated the cumulative exposure of the MWF metric of interest as a categorical variable defined by the exposure distribution of the cohort (e.g., 0, >0–50th, and ≥50th percentile of exposure). The separate models for straight, soluble, and synthetic MWF exposure included the other two MWF types as linear covariates. The model for oil-based MWF included synthetic MWF as a linear covariate; the model for water-based MWF included straight MWF as a linear covariate. We tested the trend across categories using the mean for each category and treating exposure as a continuous variable. For the two outcomes with sufficient number of cases—breast cancer and lung cancer—we examined the shape of exposure-response relations using penalized splines because exposure-response trends may be sensitive to the category cut points (R, version 2.13.1; R Development Core Team, Vienna, Austria) [11, 33].
Results
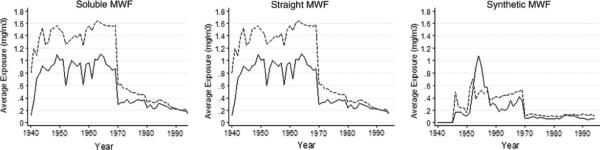
The demographic characteristics of the female cohort are shown in Table 1. The interquartile ranges of the cumulative exposure metrics were much lower than those previously reported in the combined male and female cancer incidence analyses [3]. Thus, we explored the differences in exposure levels across time between the male and female autoworkers visually by comparing the average annual exposure level for employed subjects by gender (Fig. 1). Female autoworkers generally had half to two-thirds of the average exposure levels of male autoworkers for straight and soluble MWFs. Gender differences in average exposure levels were smaller for synthetic MWF; in fact, women had higher average exposures during the 1950s.
Table 1.
Demographic and exposure characteristics of the female autoworkers cohort, 1941–2004
| Characteristic | Cohorta |
|---|---|
| Number of females | 4,825 |
| Age (years)a, median (IQR) | 59 (51–72) |
| Year of birth, median (IQR) | 1943 (1925–1952) |
| White race (%) | 73 |
| No. person-years | 139,106 |
| Years employed, median (IQR) | 17 (11–20) |
| Year of hire, median (IQR) | 1973 (1953–1977) |
| MWF type | |
| Straight MWF, % exposed | 36 % |
| Cum. exposure, median (IQR)b | 0.3 (0.07–0.8) |
| Soluble MWF, % exposed | 61 % |
| Cum. exposure, median (IQR) | 1.1 (0.4–2.8) |
| Synthetic MWF, % exposed | 26% |
| Cum. exposure, median (IQR) | 0.2 (0.05–0.6) |
| Oil-based MWF, % exposed | 62% |
| Cum. exposure, median (IQR) | 0.5 (0.2–1.3) |
| Water-based MWF, % exposed | 61 % |
| Cum. exposure, median (IQR) | 0.3 (0.1–0.8) |
Age at death or the end of follow-up (31 December 2004)
Median and IQR of exposed workers, in mg m−3, lagged 10 years
Fig. 1.
Average annual exposure levels of exposed subjects by year and MWF type for male (dashed lines) and female (solid lines) autoworkers
The female autoworkers had an overall deficit of all deaths (SMR, 0.82; 95 % CI, 0.77–0.87), but had a slightly elevated excess of mortality from cancer (all sites) (SMR, 1.10; 95 % CI, 0.98–1.22) (Table 2). Among specific sites, only lung cancer mortality was statistically significantly elevated (SMR, 2.08; 95 % CI, 1.71–2.52). Nonsignificant excesses greater than 1.25 were observed for brain cancer (SMR, 1.30; 95 %CI, 0.56–2.56), stomach cancer (SMR, 1.55; 95 % CI, 0.71–2.94), and pancreas cancer (SMR, 1.42; 95 % CI, 0.89–2.14).
Table 2.
SMRs for selected cancer sites for female autoworkers in the UAW–GM cohort for time period 1980–2004
| Cause | Mortality |
||||
|---|---|---|---|---|---|
| Obs. | Exp.a | SMR | (95 % CI) | ICD codesb | |
| All causes | 1,059 | 1,299 | 0.82 | (0.77, 0.87) | All |
| All cancers | 317 | 289 | 1.10 | (0.98,1.22) | ICD9:140-208; ICD10:C0-C97 |
| COPD | 57 | 49 | 1.16 | (0.87,1.50) | ICD9:490-496; ICD10:J40-47) |
| Ischemic heart disease | 201 | 187 | 1.08 | (0.93,1.24) | ICD9:410-414; ICD10:I20-25 |
| Site-specific cancers | |||||
| Bladder | 3 | 4 | 0.70 | (0.14, 2.05) | ICD9:188; ICD10:C67 |
| Brain | 8 | 6 | 1.30 | (0.56, 2.56) | ICD9:191; IC10:C71 |
| Breast | 43 | 42 | 1.03 | (0.75, 1.39) | ICD9:174; ICD10:C50 |
| Colon | 19 | 28 | 0.69 | (0.41, 1.07) | ICD9:153; ICD10:C18 |
| Esophagus | 3 | 4 | 0.86 | (0.17, 2.50) | ICD9:150; ICD10:C15 |
| Kidney | 4 | 5 | 0.81 | (0.22, 2.06) | ICD9:189;ICD10:C64 |
| Larynx | 1 | 1 | 0.75 | (0.01, 4.16) | ICD9:189; ICD10:C64 |
| Leukemia | 19 | 16 | 1.22 | (0.73, 1.90) | ICD9:203-208; ICD10:C90-95 |
| Liver | 2 | 4 | 0.54 | (0.06, 1.94) | ICD9:155; ICD10:C22 |
| Lung | 106 | 51 | 2.08 | (1.71, 2.52) | ICD9:162; ICD10:C34 |
| Malignant melanoma | 3 | 2 | 1.26 | (0.25, 3.67) | ICD9:172; ICD10:C43 |
| Non-Hodgkin lymphoma | 9 | 9 | 1.05 | (0.48, 2.00) | ICD9:200,202; ICD10:C82-85 |
| Ovary | 19 | 16 | 1.22 | (0.73, 1.90) | ICD9:183;ICD10:C56 |
| Pancreas | 22 | 16 | 1.42 | (0.89, 2.14) | ICD9:157; ICD10:C25 |
| Rectum | 4 | 4 | 0.90 | (0.24, 2.31) | ICD9:154; ICD10:C19-21 |
| Stomach | 9 | 6 | 1.55 | (0.71, 2.94) | ICD9:151; ICD10:C16 |
| Uterus | 2 | 3 | 0.59 | (0.07, 2.12) | ICD9:182,ICD10:C54 |
CI confidence interval, COPD chronic obstructive pulmonary disease, exp expected number of cases, ICD international classification of disease, Obs observed number of cases
Rounded to whole numbers, based on race-adjusted, female-specific Michigan rates
Based on ICD-O-03
The internal comparisons by cancer site and exposure metric are shown in Table 3. The only statistically significant elevated hazard ratio (HR) was observed for colon cancer risk in the highest category of straight MWF exposure (HR, 3.1; 95 % CI, 1.2–8.0; ptrend = 0.02). Elevated HRs with suggestive exposure-response trends were observed for brain cancer with synthetic MWF exposure (ptrend = 0.11) and for leukemia with straight MWF exposure (ptrend = 0.15), with HRs in the highest exposure category of 4.1 and 2.0, respectively. We observed protective trends for ovarian cancer with all MWF metrics (ptrend <0.01) except synthetic MWF (ptrend 0.24). No associations were found with breast cancer or lung cancer in either categorical analyses (Table 3) or nonparametric analyses using smoothing splines (not shown).
Table 3.
Categorical exposure–response relations for selected mortality cancer sites in female UAW–GM autoworkers and cumulative exposure to oil- and water-based MWF using Cox regression models for time period 1941–2004, with covariates for race, hire date, calendar year, and other MWF exposures (as linear terms)
| Mortality cancer site | Oil-based MWFa (straight + 0.3 * soluble) |
Water-based MWFb (synthetic + 0.2 * soluble) |
Straight MWF |
Soluble MWF |
Synthetic MWF |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | HR | 95 % CI | N | HR | 95 % CI | N | HR | 95 % CI | N | HR | 95 % CI | N | HR | 95 % CI | |
| Brain | |||||||||||||||
| Unexposed | 3 | 1.0 | 3 | 1.0 | 6 | 1.0 | 3 | 1.0 | 7 | 1.0 | |||||
| >0 to <median | 7 | 3.6 | 0.9–15 | 5 | 2.9 | 0.7–13 | 4 | 3.7 | 0.9–16 | 5 | 3.4 | 0.8–15 | 1 | 1.8 | 0.2–20 |
| ≥median | 0 | – | 2 | 1.1 | 0.2–6.7 | 0 | – | 2 | 1.2 | 0.2–7.3 | 2 | 4.1 | 0.7–23 | ||
| Trend p value: | – | 0.73 | – | 0.65 | 0.11 | ||||||||||
| Breast | |||||||||||||||
| Unexposed | 22 | 1.0 | 22 | 1.0 | 33 | 1.0 | 22 | 1.0 | 45 | 1.0 | |||||
| >0 to <median | 12 | 0.7 | 0.4–1.6 | 15 | 0.9 | 0.5–1.8 | 4 | 0.5 | 0.1–1.2 | 13 | 0.9 | 0.4–1.8 | 3 | 0.5 | 0.2–1.6 |
| ≥median | 19 | 0.9 | 0.5–1.6 | 16 | 0.8 | 0.4–1.4 | 16 | 1.4 | 0.7–2.5 | 18 | 0.9 | 0.5–1.7 | 5 | 0.5 | 0.2–1.4 |
| Trend p value: | 0.81 | 0.34 | 0.18 | 0.74 | 0.23 | ||||||||||
| Colon | |||||||||||||||
| Unexposed | 5 | 1.0 | 7 | 1.0 | 10 | 1.0 | 7 | 1.0 | 18 | 1.0 | |||||
| >0 to <median | 7 | 2.1 | 0.7–6.8 | 9 | 1.9 | 0.7–5.2 | 3 | 1.2 | 0.3–4.5 | 6 | 1.5 | 0.5–4.4 | 2 | 1.0 | 0.2–4.3 |
| ≥median | 10 | 2.1 | 0.7–6.2 | 6 | 0.8 | 0.3–2.5 | 9 | 3.1 | 1.2–8.0 | 9 | 1.4 | 0.5–3.8 | 2 | 0.5 | 0.1–2.2 |
| Trend p value: | 0.41 | 0.95 | 0.02 | 0.70 | 0.38 | ||||||||||
| Leukemia | |||||||||||||||
| Unexposed | 7 | 1.0 | 8 | 1.0 | 11 | 1.0 | 8 | 1.0 | 15 | 1.0 | |||||
| >0 to <median | 8 | 1.7 | 0.6–4.8 | 8 | 1.6 | 0.6–1.4 | 4 | 1.4 | 0.8–5.3 | 8 | 1.7 | 0.7–4.6 | 3 | 1.9 | 0.5–7.0 |
| ≥median | 6 | 0.7 | 0.2–2.3 | 5 | 0.6 | 0.2–2.0 | 6 | 2.0 | 0.8–5.3 | 5 | 0.5 | 0.2–1.7 | 3 | 1.3 | 0.4–4.4 |
| Trend p value: | 0.32 | 0.25 | 0.15 | 0.14 | 0.82 | ||||||||||
| Lung | |||||||||||||||
| Unexposed | 42 | 1.0 | 45 | 1.0 | 71 | 1.0 | 46 | 1.0 | 80 | 1.0 | |||||
| >0 to <median | 34 | 1.1 | 0.7–1.8 | 28 | 0.9 | 0.6–1.4 | 19 | 0.9 | 0.6–1.6 | 29 | 1.0 | 0.6–1.6 | 12 | 1.1 | 0.6–2.1 |
| ≥median | 35 | 0.8 | 0.5–1.4 | 38 | 1.0 | 0.6–1.6 | 21 | 1.0 | 0.6–1.6 | 36 | 0.9 | 0.6–1.5 | 19 | 1.4 | 0.8–2.3 |
| Trend p–value: | 0.35 | 0.85 | 0.80 | 0.74 | 0.27 | ||||||||||
| Ovary | |||||||||||||||
| Unexposed | 16 | 1.0 | 16 | 1.0 | 19 | 1.0 | 16 | 1.0 | 23 | 1.0 | |||||
| >0 to <median | 5 | 0.4 | 0.2–1.2 | 6 | 0.5 | 0.2–1.4 | 5 | 0.9 | 0.3–2.3 | 5 | 0.5 | 0.2–1.3 | 1 | 0.4 | 0.05–2.8 |
| ≥median | 5 | 0.3 | 0.1–0.7 | 4 | 0.2 | 0.1–0.7 | 2 | 0.3 | 0.1–1.0 | 5 | 0.3 | 0.1–0.7 | 2 | 0.5 | 0.2–1.4 |
| Trend p value: | 0.009 | 0.02 | 0.05 | 0.01 | 0.24 | ||||||||||
| Pancreas | |||||||||||||||
| Unexposed | 11 | 1.0 | 11 | 1.0 | 14 | 1.0 | 11 | 1.0 | 18 | 1.0 | |||||
| >0 to <median | 6 | 0.7 | 0.3–2.1 | 5 | 0.7 | 0.2–2.0 | 4 | 1.0 | 0.3–3.1 | 6 | 0.8 | 0.3–2.4 | 1 | 0.5 | 0.1–3.4 |
| ≥median | 6 | 0.5 | 0.2–1.3 | 7 | 0.7 | 0.3–1.9 | 5 | 1.1 | 0.4–3.1 | 6 | 0.6 | 0.2–1.5 | 4 | 1.3 | 0.5–3.9 |
| Trend p value: | 0.80 | 0.71 | 0.86 | 0.27 | 0.53 | ||||||||||
| Stomach | |||||||||||||||
| Unexposed | 5 | 1.0 | 6 | 1.0 | 7 | 1.0 | 6 | 1.0 | 9 | 1.0 | |||||
| >0 to <median | 4 | 1.2 | 0.3–4.5 | 3 | 0.8 | 0.2–3.0 | 2 | 1.0 | 0.2–5.3 | 3 | 0.9 | 0.2–3.6 | 0 | – | |
| ≥median | 1 | 0.2 | 0.02–1.2 | 1 | 0.1 | 0.01–1.3 | 1 | 0.3 | 0.1–2.0 | 1 | 0.1 | 0.01–1.0 | 1 | 0.5 | 0.06–1.1 |
| Trend p value: | 0.03 | 0.08 | 0.22 | 0.04 | 0.5 | ||||||||||
CI confidence interval, HR hazard ratio, MWF metalworking fluids
Median cumulative oil-based MWF = 0.55 mg m−3
Median cumulative water-based MWF = 0.33 mg m−3
Discussion
This study examined the risk of cancer mortality in a moderately sized cohort of female autoworkers, with six decades of follow up. The female autoworkers had an overall excess of cancers (SMR, 1.10), and in particular lung cancer (SMR, 2.08), compared to the reference group of Michigan women. Because the overall deficit of deaths (SMR = 0.82) in this cohort suggests a healthy worker hire effect, we examined too the risk of cancer in internal comparisons using an incident hire population followed over the full 60 year study period in relation to several metrics of MWF exposure. We observed an increased risk of colon cancer mortality with straight MWF exposure; however, we also observed few cases of malignant melanoma and cancers of the bladder, rectal, and larynx (which have been strongly associated with MWF exposure in previous studies [2, 3, 5, 12, 14, 34–39]) to examine exposure–response relations for these tumors. For all but lung and breast cancer, the number of cancer outcomes remained too sparse to examine exposure-response relations with greater than two exposure categories. In addition, the female autoworkers' exposure levels were generally much lower than the male autoworkers' exposures, despite having a similar, moderate prevalence of exposure and duration of employment (males: median, 18 years; females: median, 17 years). In a recently published study of malignant melanoma in this autoworkers cohort, the comparative medians (IQRs) for white males were 0.7 (2.5) for straight MWF, 4.4 (9.2) for soluble MWF, and 0.5 (1.6) for synthetic [33]. The corresponding numbers for women reported in this paper were much lower: 0.3 (0.73) for straight MWF, 1.1 (2.4) for soluble MWF, and 0.2 (0.55) for synthetic MWF. Thus, these analyses provide estimates of the risk associated at the low end of the MWF exposure scale.
Among these female autoworkers, the risk of colon cancer was associated with straight MWFs. In contrast, a previous analysis of colon cancer incidence for both genders combined found a stronger association with waterbased MWFs [3]. However, analyses of rectal cancer mortality (which may have similar risk factors) for both genders combined found stronger risks with straight MWFs but also observed elevated risks with soluble and synthetic MWFs [6]. The inconsistency by MWF metric may suggest that the MWF metrics are surrogates for other causal agents for colon cancer risk, such as tramp oils, abrasive materials, and metal particulates from the grinding and machining processes, which are present with any MWF exposure.
Although we observed an elevated lung cancer risk in external comparisons, in the internal comparisons the lung cancer mortality rate was not associated with any MWF exposure metric. The excess mortality rate for lung cancer may be attributable to differences in the smoking patterns of this cohort compared to Michigan women, but we could not adjust for smoking in these analyses. Previous studies have shown that the smoking rates for women can vary by employment status and type of work, and thus, internal comparisons are necessary [21]. The slightly elevated rates of chronic obstructive pulmonary disease and ischemic heart disease mortality provide additional support for smoking as a primary role in the elevated lung cancer SMR. Previous mortality and incidence studies of the UAW–GM cohort have observed a protective effect with lung cancer and water-based MWF exposure (a likely surrogate for endotoxin exposure) in analyses that included men and women [3, 7]. In this study, we found no evidence for a protective effect with lung cancer, but our ability to detect this association may have been limited by the lower exposure levels for water-based MWF in this female cohort compared to the incidence cohort (interquartile range, 75th percentile–25th percentile: 0.7 vs. 2.8, respectively) [3].
In this study, there was limited evidence of an increased risk of breast cancer mortality with MWF exposure. In contrast, a previous nested case–control study of female autoworkers in this cohort was modestly positive for soluble MWF [10]. There were several differences between the present study and the earlier study of breast cancer in this cohort. The previous study focused solely on breast cancer and found the strongest association with exposure to soluble fluid occurring in the decade prior to disease date (incidence (n = 86) or mortality (n = 13)). The goal of the current analysis was to estimate the association between oil- and water-based fluid and death from several different cancers in women; thus, we applied a 10-year lag in exposure to all analyses to account for latency. By design, the current analysis did not replicate the previously reported association; however, the HR for breast cancer was weakly elevated in the highest exposure category for straight MWF, which provides modest support for the association with oil-based fluids.
Although a protective effect of ovarian cancer was observed with water-based MWF exposure, we suspect that the association seen here may represent unmeasured confounding from behavioral differences in the study population. Ovarian cancer risk has been previously associated with differences in oral contraceptive use and the number of pregnancies [40]. Women with longer employment duration or who worked in the more skilled operations (e.g., machining or grinding), and thus achieve higher cumulative exposures, may have different patterns of oral contraceptives use or reproductive histories than their colleagues with interrupted employment patterns or in the lower exposed unskilled operations (e.g., assembly work). The employment patterns differed for the unexposed female autoworkers compared to the highest exposed female autoworkers (mean employment duration, 23.5 vs. 26.5, respectively). These differences may be attributable to reproductive history patterns, since working women generally have lower parity and higher age at first birth than nonworking women [21]. However, as is common in retrospective industry-based studies, we were unable to obtain information on nonoccupational risk factors and thus cannot adjust for confounding by important reproductive or other behavioral risk factors. Other challenges in examining cancer risk in women, including difficulty in outcome ascertainment due to name changes or diagnostic misclassification on death certificates [21], are less likely to be correlated with exposure patterns.
Evaluating the associations with overlapping components of oil- and water-based MWF components was an innovation of this study. However, these analyses assumed constant weights for the contribution of soluble MWF that were chosen from the best fitting models in previous analyses [3]. Incorrect weights would introduce exposure misclassification and limit our ability to see associations. Our results using these weights, however, were consistent with the findings from analyses that considered each MWF type on its own.
In summary, this study provides evidence of an association between colon cancer and straight MWF exposure. Because of the limited number of cases for these cancer sites and the much lower MWF exposure levels experienced by the women compared to the men, we were unable to test whether there were gender differences in risk; however, this study does provide estimates of the risk at the lower end of the exposure–response scale. Evaluating the risks separately for women allowed us to examine potential associations with cancers of the reproductive system; however, future studies of women's occupational cancer risks need to collect information on important risk factors, especially reproductive histories.
Acknowledgments
This work was supported by Grant R01 OH008927 from the National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention, Department of Health and Human Services.
References
- 1.Tolbert PE. Oils and cancer. Cancer Causes Control. 1997;8:386–405. doi: 10.1023/a:1018409422050. [DOI] [PubMed] [Google Scholar]
- 2.Friesen MC, Costello S, Eisen EA. Quantitative exposure to metalworking fluids and bladder cancer incidence in a cohort of autoworkers. Am J Epidemiol. 2009;169:1471–1478. doi: 10.1093/aje/kwp073. [DOI] [PubMed] [Google Scholar]
- 3.Friesen MC, Costello S, Thurston SW, Eisen EA. Distinguishing the common components of oil- and water-based metalworking fluids for assessment of cancer incidence risk in autoworkers. Am J Ind Med. 2011;54:450–460. doi: 10.1002/ajim.20932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costello S, Friesen MC, Christiani DC, Eisen EA. Metalworking fluids and malignant melanoma in autoworkers. Epidemiology. 22:90–97. doi: 10.1097/EDE.0b013e3181fce4b8. [DOI] [PubMed] [Google Scholar]
- 5.Zhao Y, Krishnadasan A, Kennedy N, Morgenstern H, Ritz B. Estimated effects of solvents and mineral oils on cancer incidence and mortality in a cohort of aerospace workers. Am J Ind Med. 2005;48:249–258. doi: 10.1002/ajim.20216. [DOI] [PubMed] [Google Scholar]
- 6.Malloy EJ, Miller KL, Eisen EA. Rectal cancer and exposure to metalworking fluids in the automobile manufacturing industry. Occup Environ Med. 2007;64:244–249. doi: 10.1136/oem.2006.027300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta AJ, Malloy EJ, Applebaum KM, Schwartz J, Christiani DC, Eisen EA. Reduced lung cancer mortality and exposure to synthetic fluids and biocide in the auto manufacturing industry. Scand J Work Environ Health. 2010;36:499–508. doi: 10.5271/sjweh.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agalliu I, Eisen EA, Kriebel D, Quinn MM, Wegman DH. A biological approach to characterizing exposure to metalworking fluids and risk of prostate cancer (United States) Cancer Causes Control. 2005;16:323–331. doi: 10.1007/s10552-004-4323-7. [DOI] [PubMed] [Google Scholar]
- 9.Agalliu I, Kriebel D, Quinn MM, Wegman DH, Eisen EA. Prostate cancer incidence in relation to time windows of exposure to metalworking fluids in the auto industry. Epidemiology. 2005;16:664–671. doi: 10.1097/01.ede.0000173266.49104.bb. [DOI] [PubMed] [Google Scholar]
- 10.Thompson D, Kriebel D, Quinn MM, Wegman DH, Eisen EA. Occupational exposure to metalworking fluids and risk of breast cancer among female autoworkers. Am J Ind Med. 2005;47:153–160. doi: 10.1002/ajim.20132. [DOI] [PubMed] [Google Scholar]
- 11.Thurston SW, Eisen EA, Schwartz J. Smoothing in survival models: an application to workers exposed to metalworking fluids. Epidemiology. 2002;13:685–692. doi: 10.1097/00001648-200211000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Savitz DA. Epidemiologic evidence on the carcinogenicity of metalworking fluids. Appl Occup Environ Hyg. 2003;18:913–920. doi: 10.1080/10473220390237539. [DOI] [PubMed] [Google Scholar]
- 13.Health) NNIfOS . Occupational exposure to metalworking fluids. In: Services UDoHH, editor. Criteria for a recommended standard. Public Health Service CDC; Cincinnati OH: 1998. [Google Scholar]
- 14.Eisen EA, Bardin J, Gore R, Woskie SR, Hallock MF, Monson RR. Exposure-response models based on extended follow-up of a cohort mortality study in the automobile industry. Scand J Work Environ Health. 2001;27:240–249. doi: 10.5271/sjweh.611. [DOI] [PubMed] [Google Scholar]
- 15.Eisen EA, Tolbert PE, Monson RR, Smith TJ. Mortality studies of machining fluid exposure in the automobile industry I: a standardized mortality ratio analysis. Am J Ind Med. 1992;22:809–824. doi: 10.1002/ajim.4700220604. [DOI] [PubMed] [Google Scholar]
- 16.Bardin JA, Eisen EA, Tolbert PE, et al. Mortality studies of machining fluid exposure in the automobile industry. V: a case–control study of pancreatic cancer. Am J Ind Med. 1997;32:240–247. doi: 10.1002/(sici)1097-0274(199709)32:3<240::aid-ajim9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 17.Arbuckle TE. Are there sex and gender differences in acute exposure to chemicals in the same setting? Environ Res. 2006;101:195–204. doi: 10.1016/j.envres.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy SM, Koehoorn M. Exposure assessment in epidemiology: does gender matter? Am J Ind Med. 2003;44:576–583. doi: 10.1002/ajim.10297. [DOI] [PubMed] [Google Scholar]
- 19.Messing K, Mager StellmanJ. Sex, gender and women's occupational health: the importance of considering mechanism. Environ Res. 2006;101:149–162. doi: 10.1016/j.envres.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 20.Messing K, Punnett L, Bond M, et al. Be the fairest of them all: challenges and recommendations for the treatment of gender in occupational health research. Am J Ind Med. 2003;43:618–629. doi: 10.1002/ajim.10225. [DOI] [PubMed] [Google Scholar]
- 21.Zahm SH, Blair A. Occupational cancer among women: where have we been and where are we going? Am J Ind Med. 2003;44:565–575. doi: 10.1002/ajim.10270. [DOI] [PubMed] [Google Scholar]
- 22.Zahm SH, Pottern LM, Lewis DR, Ward MH, White DW. Inclusion of women and minorities in occupational cancer epidemiologic research. J Occup Med. 1994;36:842–847. [PubMed] [Google Scholar]
- 23.Gray J, Evans N, Taylor B, Rizzo J, Walker M. State of the evidence: the connection between breast cancer and the environment. Int J Occup Environ Health. 2009;15:43–78. doi: 10.1179/107735209799449761. [DOI] [PubMed] [Google Scholar]
- 24.Shanle EK, Xu W. Endocrine disrupting chemicals targeting estrogen receptor signaling: identification and mechanisms of action. Chem Res Toxicol. 2011;24:6–19. doi: 10.1021/tx100231n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fu JB, Kau TY, Severson RK, Kalemkerian GP. Lung cancer in women: analysis of the national surveillance, epidemiology, and end results database. Chest. 2005;127:768–777. doi: 10.1378/chest.127.3.768. [DOI] [PubMed] [Google Scholar]
- 26.Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers: a different disease. Nat Rev Cancer. 2007;7:778–790. doi: 10.1038/nrc2190. [DOI] [PubMed] [Google Scholar]
- 27.Fasco MJ, Hurteau GJ, Spivack SD. Gender-dependent expression of alpha and beta estrogen receptors in human non-tumor and tumor lung tissue. Mol Cell Endocrinol. 2002;188:125–140. doi: 10.1016/s0303-7207(01)00750-x. [DOI] [PubMed] [Google Scholar]
- 28.Siegfried JM. Women and lung cancer: does oestrogen play a role? Lancet Oncol. 2001;2:506–513. doi: 10.1016/S1470-2045(01)00457-0. [DOI] [PubMed] [Google Scholar]
- 29.Applebaum KM, Malloy EJ, Eisen EA. Left truncation, susceptibility, and bias in occupational cohort studies. Epidemiology. 2011;22:599–606. doi: 10.1097/EDE.0b013e31821d0879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hallock MF, Smith TJ, Woskie SR, Hammond SK. Estimation of historical exposures to machining fluids in the automotive industry. Am J Ind Med. 1994;26:621–634. doi: 10.1002/ajim.4700260505. [DOI] [PubMed] [Google Scholar]
- 31.Woskie SR, Smith TJ, Hammond SK, et al. Factors affecting worker exposures to metal-working fluids during automotive component manufacturing. Appl Occup Environ Hyg. 1994;9:612–621. [Google Scholar]
- 32.Betenia N, Costello S, Eisen EA. Risk of cervical cancer in female autoworkers exposed to metalworking fluids. Scand J Work Environ Health. 2012;38:78–83. doi: 10.5271/sjweh.3193. [DOI] [PubMed] [Google Scholar]
- 33.Eisen EA, Agalliu I, Thurston SW, Coull BA, Checkoway H. Smoothing in occupational cohort studies: an illustration based on penalised splines. Occup Environ Med. 2004;61:854–860. doi: 10.1136/oem.2004.013136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eisen EA, Tolbert PE, Hallock MF, Monson RR, Smith TJ, Woskie SR. Mortality studies of machining fluid exposure in the automobile-industry.3. A case-control study of larynx cancer. Am J Ind Med. 1994;26:185–202. doi: 10.1002/ajim.4700260205. [DOI] [PubMed] [Google Scholar]
- 35.Sullivan PA, Eisen EA, Woskie SR, et al. Mortality studies of metalworking fluid exposure in the automobile industry: VI. A case-control study of esophageal cancer. Am J Ind Med. 1998;34:36–48. doi: 10.1002/(sici)1097-0274(199807)34:1<36::aid-ajim6>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 36.Tolbert PE, Eisen EA, Pothier LJ, Monson RR, Hallock MF, Smith TJ. Mortality studies of machining-fluid exposure in the automobile industry. II. Risks associated with specific fluid types. Scand J Work Environ Health. 1992;18:351–360. doi: 10.5271/sjweh.1562. [DOI] [PubMed] [Google Scholar]
- 37.Zeka A, Eisen EA, Kriebel D, Gore R, Wegman DH. Risk of upper aerodigestive tract cancers in a case-cohort study of autoworkers exposed to metalworking fluids. Occup Environ Med. 2004;61:426–431. doi: 10.1136/oem.2003.010157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silverman DT, Levin LI, Hoover RN, Hartge P. Occupational risks of bladder cancer in the United States: I. White men. J Natl Cancer Inst. 1989;81:1472–1480. doi: 10.1093/jnci/81.19.1472. [DOI] [PubMed] [Google Scholar]
- 39.Costello S, Friesen MC, Christiani DC, Eisen EA. Metalworking fluids and malignant melanoma in autoworkers. Epidemiology. 2011;22:90–97. doi: 10.1097/EDE.0b013e3181fce4b8. [DOI] [PubMed] [Google Scholar]
- 40.Salehi F, Dunfield L, Phillips KP, Krewski D, Vanderhyden BC. Risk factors for ovarian cancer: an overview with emphasis on hormonal factors. J Toxicol Environ Health B Crit Rev. 2008;11:301–321. doi: 10.1080/10937400701876095. [DOI] [PubMed] [Google Scholar]