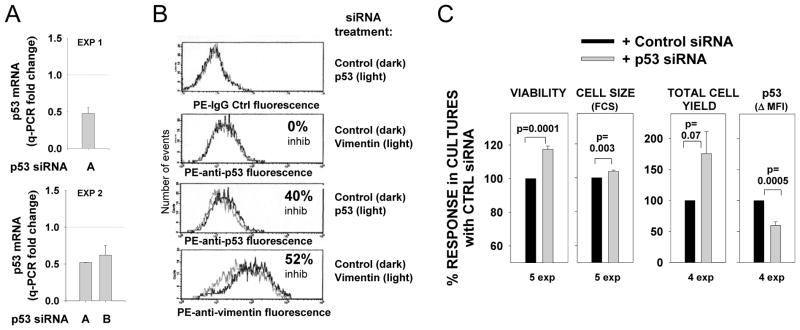
FIGURE 6. p53 siRNA treatment of pre-activated human FO cells diminishes p53 protein expression but augments B cell viability, cell size and total cell yield.
Purified FO cells from tonsils or spleens were pre-activated for 2 d (with either BCR:CD21-L, BCR:CD21-L + IL-4, or BCR:CD21-L + IL-4 + BAFF) prior to nucleofection with p53 siRNA, control siRNA (or vimentin siRNA) as described in materials and methods. (A) mRNA was isolated from cells at 24–32 hours post nucleofection. cDNA was prepared and analyzed for p53 and actin levels by q-PCR with specific primers. Values for Δ Ct values were obtained as described for Figure 3A; fold-change was calculated by comparing Δ Ct values within p53 siRNA-treated cultures with respective Δ Ct values in cultures treated with control siRNA. Shown are fold-change values from transfections performed with two distinct activated B cell populations and two different sources of p53 siRNA (mean +/−SEM values from replicate qPCR assays are shown). (B) Cells were harvested at 3 days following nucleofection with the indicated siRNA and fixed, permeabilized and stained intracellularly with PE-anti-p53 antibody, PE-anti-vimentin, or PE-IgG control. Shown is PE fluorescence histograms on a 4-log scale. (C) At 3 to 4 days after nucleofection, fixed cells were analyzed for cell viability (by light scatter), cell size (by FSC of viable subset), and total cell yield (as determined with standardization beads, or in the first experiment, by comparison of “events per minute” during acquisition of cells from control and p53 siRNA-treated cultures). To standardize data from multiple experiments, values for each parameter within an experiment were expressed as a % of the value obtained with control siRNA-treated cells. Shown are the mean ± SEM values of such values. A two-tailed, non-paired Student’s t test was used to compare the responses in cultures treated with p53 siRNA versus control siRNA (p values of significance are shown).