Abstract
Mutations in the Sonic Hedgehog limb enhancer, the zone of polarizing activity regulatory sequence (LMBR1, commonly called the ZRS), cause limb malformations. In humans, three classes of mutations have been proposed based on the limb phenotype; single base changes throughout the region cause preaxial polydactyly, single base changes at one specific site cause Werner mesomelic syndrome and large duplications cause polysyndactyly. This study presents a novel mutation– a small insertion. In a Swedish family with autosomal dominant preaxial polydactyly, we found a 13 base pair insertion within the ZRS, ZRS603ins13 (NG-009240.1:g.106934_106935ins13). Computational transcription factor binding site predictions suggest that this insertion creates new binding sites and a mouse enhancer assay shows that this insertion causes ectopic gene expression. This study is the first to discover a small insertion in an enhancer that causes a human limb malformation and suggests a potential mechanism that could explain the ectopic expression caused by this mutation.
Keywords: enhancer, limb, polydactyly, SHH, LMBR1, ZRS
Preaxial polydactyly is often thought to be the result of problems in development related to patterning of the limb along the anterior-posterior (AP) axis. The AP axis is regulated by cells in a small posterior region of the limb bud called the zone of polarizing activity (ZPA). Cells in the ZPA express Sonic Hedgehog (SHH; MIM# 600725), which controls both digit number and digit identity.
Normal Shh expression level and restriction to the posterior ZPA region is governed by a long range cis-regulatory element called the ZPA regulatory sequence (ZRS; MIM# 605522). The ZRS is located about 1 megabase away from SHH, within intron 5 of the limb region 1 homolog gene (LMBR1; MIM# 605522). This region has a high degree of sequence conservation from humans to fish and controls Shh expression in the limb (Lettice, et al., 2003). Mutations in the ZRS have been found to cause preaxial polydactyly in many animals including dogs, cats, chickens and humans. These represent more than 20 different mutations over a region of approximately 2 kilobases. Experiments in mice and chickens that have preaxial polydactyly due to ZRS mutations have shown that these mutations can alter regulatory function, causing ectopic anterior expression of Shh and increased posterior mRNA expression levels. Other mutations, not found in mouse models, have been tested in a mouse enhancer assay and shown to cause anterior expression of the reporter gene (reviewed in VanderMeer and Ahituv, 2011), suggesting that anterior ZRS activity is a common mechanism in ZRS mutations.
Since the identification of the ZRS as a long-range enhancer for SHH, 13 different point mutations and 10 duplications including the ZRS have been identified in humans with limb malformations (reviewed in VanderMeer and Ahituv, 2011). These malformations represent a wide range of phenotypes. While large duplications including the ZRS and surrounding sequences cause complex Haas-type polysyndactyly (webbing between digits along with extra digits), point mutations in the ZRS cause relatively milder phenotypes including preaxial polydactyly with or without triphalangeal thumbs and Werner mesomelic syndrome.
Here we report a new mutation in the ZRS that causes preaxial polydactyly with triphalangeal thumb in a Swedish family. Previously reported preaxial polydactyly mutations have single base pair (bp) changes, but this mutation is an insertion of 13 bp in the highly conserved ZRS. Computational analyses of ZRS mutations in previous studies suggest that disruption of transcription factor binding sites (TFBS) may be a mechanism for changing AP limb patterning (Albuisson, et al., 2010; Gurnett, et al., 2007). In this case, our computational analysis suggests that rather than losing binding sites, the insertion may change patterning by creating novel TFBS for multiple transcription factors known to be critical for normal limb development. Analysis of the mutated ZRS sequence in a mouse enhancer assay shows that it causes expanded activity from the ZPA to an ectopic anterior limb region.
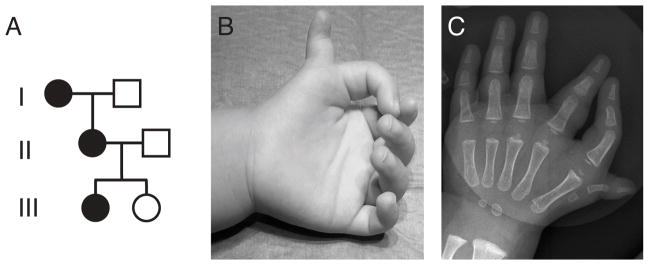
The proband (Figure 1A, individual III/1) has bilateral symmetrical triphalangeal thumbs with an extra hypoplastic radial thumb containing two phalanges and one metacarpal – preaxial polydactyly type II (PPD2; MIM# 174500) (Figure 1B, C). The triphalangeal thumb was not opposable and had a finger-like appearance. There was no involvement of the lower limbs in the proband or in any other affected family member. The proband belongs to a large 6 generation family with 78 individuals, 18 of whom are reported to be affected (Supp. Figure S1). DNA samples were available from 3 affected and 3 unaffected individuals in three generations and clinical examinations were performed on the proband’s parents and sister (Figure 1A).
Figure 1.
Family with preaxial polydactyly and triphalangeal thumb. A. Pedigree of the family showing subjects available for DNA screening. B,C. Photograph of the right hand and radiograph of the left hand of the proband (patient III/1) showing preaxial polydactyly with triphalangeal thumb.
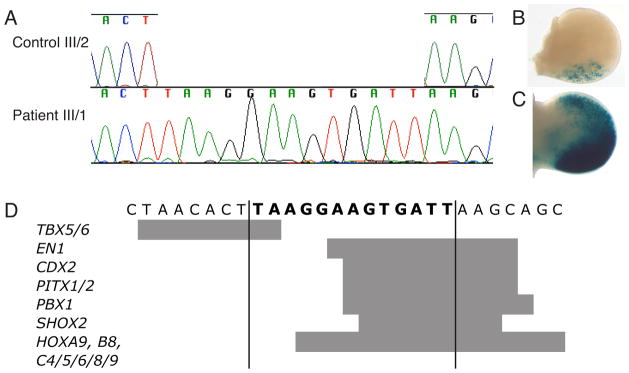
We screened a 2.1 kilobase (kb) region that encompasses the ZRS and a nearby region where mutations were shown to cause polydactyly in dogs (pZRS) (Park, et al., 2008) (chr7:156,583,564–156,585,727; UCSC Genome Browser; http://genome.ucsc.edu; hg19; g.104811–105583 in reference sequence AC007097.4). All affected individuals were found to be heterozygous for a 13 bp insertion (TAAGGAAGTGATT, Figure 2A) starting at position 603 of the ZRS sequence (Lettice, et al., 2003), position g.106934 within with reference sequence NG_009240.1 for the ZRS, here named ZRS603ins13 (approved variant nomenclature NG_009240.1:g.106934_106935ins13). This mutation has been added to the LMBR1 mutation database (www.LOVD.nl/LMBR1). None of the three unaffected members tested (Figure 1A; individuals I/2, II/2, III/2) carried this insertion.
Figure 2.
The insertion changes expression of a reporter gene and creates predicted transcription factor binding sites. A. Chromatogram of the ZRS603ins13 (NG_009240.1:g.106934_106935ins13) insertion mutation. Sequencing a cloned allele of the ZRS from patient III/1 shows an insertion of 13 bp compared to the wildtype sequence represented by the sequence from an unaffected sibling (III/2). B. ZRS E11.5 mouse transgenic assay of human wtZRS showing posterior LacZ expression . C. ZRS E11.5 mouse transgenic assay of ZRS603ins13 showing strong posterior and ectopic anterior LacZ expression. D. The 13bp insertion, in bold, creates binding sites for multiple limb-associated transcription factors. Grey bars indicate the location of the TFBS.
To understand how this insertion could affect the expression of SHH in the developing limb, we screened the mutant ZRS sequence for TFBS differences from the wildtype human ZRS sequence. TFBS motifs from the UniPROBE database (Newburger and Bulyk, 2009) as well as TFBS compiled from the literature for important limb developmental transcription factors not represented in the database were used for this screen (see Supp. Methods). The insertion creates two major sites that match the binding preferences for several transcription factors, including some that are important in limb development (Figure 2D, Supp. Figure S2). Among the detected TFBS known to be involved in limb development, are Engrailed1 (EN1), TBX5, TBX6, SOX8, and multiple HOX genes including HOXA9, HOXB8, and HOXC genes expressed in the developing limb (Nelson, et al., 1996) (Figure 2D). No limb-related TFBS were predicted to be disrupted by the insertion
We next set out to determine the effect of the ZRS603ins13 insertion on the ZPA-specific enhancer function of the ZRS using a transgenic mouse enhancer assay. Wildtype and mutant alleles of the 2.1 kb human ZRS and pZRS region were cloned into the HSP68-LacZ enhancer assay vector. This vector has an Hsp68 minimal promoter (a promoter that is not sufficient to drive reporter expression without the presence of a functional enhancer) followed by the LacZ reporter gene. Transgenic mice carrying these vectors were screened at mouse embryonic (E) day 11.5 – the stage at which previous studies have seen ectopic reporter gene expression from ZRS mutation assays (see Supp. Methods). The wildtype ZRS sequence recapitulates the normal Shh expression pattern in E11.5 mouse embryos with expression of the reporter gene in the posterior region of the limb bud in seven out of eight independent transgenic mice (Figure 2B, Supp. Figure S3). With the mutation, four out of six LacZ-positive embryos had limb expression and all four of these embryos showed ectopic anterior expression (Figure 2C, Supp. Figure S3) instead of the normal posterior-restricted ZPA expression pattern (Figure 2B). The expression is limited to the mesoderm of the limb bud and absent from the apical ectoderma ridge (AER) with staining at the anterior and posterior sides. While the four embryos did not show the same intensity of staining (Supp. Figure S3), each embryo had anterior expression that was nearly as strong as the posterior expression. It is worth noting that two of the seven limb-expressing wildtype ZRS embryos also showed a small degree of anterior limb expression that was much weaker than the posterior expression, in contrast to the ZRS603ins13 embryos where the anterior and posterior expression were similar (Supp. Figure S3). The variation between embryos is likely due to integration site or copy number differences between embryos and these differences highlight the qualitative nature of such transgenic assays. The reproducible anterior expression in ZRS603ins13 embryos is consistent with the enhancer expression pattern observed in similar assays with ZRS mutations that have been shown to cause polydactyly (Furniss, et al., 2008; Lettice, et al., 2003; Lettice, et al., 2008). No other consistent expression patterns were observed in these embryos.
These findings indicate that g.106934_106935ins13 (ZRS603ins13), the small insertion mutation within the ZRS, is likely the cause of preaxial polydactyly and triphalangeal thumb in this family. The mutation is shown to be present in three generations of affected individuals in a fully penetrant inheritance pattern with an invariable phenotype. This inheritance pattern has also been seen with most other ZRS mutations (Albuisson, et al., 2010; Farooq, et al., 2010; Semerci, et al., 2009). However some families with ZRS point mutations have variable phenotypes among affected individuals (Gurnett, et al., 2007; Lettice, et al., 2003) and at least one family is known to have reduced penetrance with phenotypically normal carriers (Gurnett, et al., 2007).
The known human point mutations in the ZRS are dispersed over approximately 600 bp and cause a range of preaxial polydactyly phenotypes (VanderMeer and Ahituv, 2011).There is no clear relationship between the location of the mutation and the severity of the phenotype, but it its thought that the mutations may disrupt TFBS. The ZRS603ins13 mutation results in the creation of two motifs that are predicted to bind multiple transcription factors that are known to be expressed in early limb development including TBX5, TBX6, Engrailed1 (EN1), SOX8, and multiple HOX genes. TBX5 mutations have been shown to cause Holt-Oram syndrome (MIM# 142900), a congenital defect syndrome with limb malformations that include triphalangeal thumbs. EN1 (MIM# 131290) is a homeodomain-containing transcription factor required for ventral development of the limb and its deletion in mouse models leads to AP limb patterning defects. A consensus binding site for many HOX genes that are all expressed at various stages in the developing limb bud (Nelson, et al., 1996) was also found in this insertion. While these transcription factors are not known to directly regulate SHH expression in the limb, they are present at the stages in development where abnormal binding to an important regulatory element could stimulate improper expression or interfere with the normal binding of transcription factors that should be regulating SHH. Additional limb-associated TFBS created by this mutation that were not detected by our computational analysis could exist and may be related to ZRS activity in the developing limb.
Previous studies have reported bioinformatic analyses suggesting that point mutations in the human ZRS may destroy binding sites for CDX (Gurnett, et al., 2007), MEIS1 and SOX9 (Albuisson, et al., 2010), but no studies have reported the addition of novel TFBS created by the mutation. Interestingly, an increased affinity for proteins has been seen for two mutated ZRS sequences (Farooq, et al., 2010; Zhao, et al., 2009), though the identity of the particular element(s) that bind preferentially to the mutant sequence was only shown for one case (in this case, HnRNP U; Zhao, et al., 2009). Our study did not find any limb-associated TFBS to be disrupted by the insertion, though it is possible that there are binding sites for known transcription factors that could not be detected by the TFBS prediction program used for this analysis and there are limb-associated transcription factors whose DNA binding motifs are not known.
We also show that this mutation causes the ZRS to drive expression of a reporter gene in the anterior portion of the limb bud where Shh is not normally expressed. This type of ectopic anterior expression pattern is consistent with observations in mouse and chicken models of preaxial polydactyly and has also been reported in mouse enhancer assays of human ZRS point mutations that were inserted into a mouse ZRS sequence. Additionally, the LacZ reporter expression of the g.106934_106935ins13) mutation appears to be in a larger posterior region than the normal ZPA expression. While the reporter assay used here is not a quantitative measure of enhancer activity, this increased posterior expression has been reported as a trend in similar enhancer mutation analyses. In addition, mouse and chicken models of preaxial polydactyly have been shown to have increased posterior Shh expression due to ZRS mutations (Blanc, et al., 2002; Dunn, et al., 2011). It has been suggested that the degree of ectopic expression in the mouse transgenic reporter assay is related to the severity of the human phenotype caused by the ZRS mutation (Lettice, et al., 2008). The inherent expression differences due to integration site and copy number variability makes this relationship difficult to define, but the ectopic anterior expression from this 13 bp insertion mutation appears to be stronger than what was seen with other ZRS point mutations that cause preaxial polydactyly (Furniss, et al., 2008; Lettice, et al., 2008). The relationship between mutations in the ZRS and the resulting phenotype are complicated and suggest that the regulatory potential of the ZRS is not limited to enhancer activity – driving expression of Shh in the posterior limb bud – but also may include some repressor activity to prevent the expression of Shh in the anterior limb. The mutation reported in this study and the effect it has in creating novel TFBS suggests that the binding of additional transcription factors and the ablation of TFBS and loss of transcription factor binding may lead to similar outcomes.
Supplementary Material
Acknowledgments
We thank Sigrid Sahlén and Kristina Lagerstedt for excellent technical assistance. This work was supported by the Swedish Research Council, the Swedish Society of Medicine, Foundation Frimurarna Barnhuset in Stockholm, Sällskapet Barnavård, HRH the Crown Princess Lovisa Foundation, Karolinska Institutet, and Stockholm City Council. This research was supported by the NICHD grant number R01HD059862 (NA, GB and JEV), a Stanford Graduate Fellowship (AMW), Bio-X Stanford Interdisciplinary Graduate Fellowship (AMW), and the Packard foundation (GB). NA is also supported by NHGRI grant number R01HG005058 (with GB) and NIGMS award number GM61390.
The study was approved by the Regional Ethical Review Board in Stockholm, Sweden (protocol number 2010/193032) and the UCSF Committee on Human Research (protocol number 10-03111).
Footnotes
Supporting Information for this preprint is available from the Human Mutation editorial office upon request (humu@wiley.com)
References
- Albuisson J, Isidor B, Giraud M, Pichon O, Marsaud T, David A, Le Caignec C, Bezieau S. Identification of two novel mutations in Shh long-range regulator associated with familial pre-axial polydactyly. Clin Genet. 2010;79:371–377. doi: 10.1111/j.1399-0004.2010.01465.x. [DOI] [PubMed] [Google Scholar]
- Blanc I, Bach A, Robert B. Unusual pattern of Sonic hedgehog expression in the polydactylous mouse mutant Hemimelic extra-toes. Int J Dev Biol. 2002;46:969–974. [PubMed] [Google Scholar]
- Dunn IC, Paton IR, Clelland AK, Sebastian S, Johnson EJ, McTeir L, Windsor D, Sherman A, Sang H, Burt DW, et al. The chicken polydactyly (Po) locus causes allelic imbalance and ectopic expression of Shh during limb development. Dev Dyn. 2011;240:1163–1172. doi: 10.1002/dvdy.22623. [DOI] [PubMed] [Google Scholar]
- Farooq M, Troelsen JT, Boyd M, Eiberg H, Hansen L, Hussain MS, Rehman SU, Azhar A, Ali A, Bakhtiar SM, et al. Preaxial polydactyly/triphalangeal thumb is associated with changed transcription factor-binding affinity in a family with a novel point mutation in the long-range cis-regulatory element ZRS. Eur J Hum Genet. 2010;18:733–736. doi: 10.1038/ejhg.2009.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furniss D, Lettice LA, Taylor IB, Critchley PS, Giele H, Hill RE, Wilkie AOM. A variant in the sonic hedgehog regulatory sequence (ZRS) is associated with triphalangeal thumb and deregulates expression in the developing limb. Hum Mol Genet. 2008;17:2417–2423. doi: 10.1093/hmg/ddn141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurnett CA, Bowcock AM, Dietz FR, Morcuende JA, Murray JC, Dobbs MB. Two novel point mutations in the long-range SHH enhancer in three families with triphalangeal thumb and preaxial polydactyly. Am J Med Genet. 2007;143A:27–32. doi: 10.1002/ajmg.a.31563. [DOI] [PubMed] [Google Scholar]
- Lettice LA, Heaney SJH, Purdie LA, Li L, de Beer P, Oostra BA, Goode D, Elgar G, Hill RE, de Graaff E. A long-range Shh enhancer regulates expression in the developing limb and fin and is associated with preaxial polydactyly. Hum Mol Genet. 2003;12:1725–1735. doi: 10.1093/hmg/ddg180. [DOI] [PubMed] [Google Scholar]
- Lettice LA, Hill AE, Devenney PS, Hill RE. Point mutations in a distant sonic hedgehog cis-regulator generate a variable regulatory output responsible for preaxial polydactyly. Hum Mol Genet. 2008;17:978–985. doi: 10.1093/hmg/ddm370. [DOI] [PubMed] [Google Scholar]
- Nelson CE, Morgan BA, Burke AC, Laufer E, DiMambro E, Murtaugh LC, Gonzales E, Tessarollo L, Parada LF, Tabin C. Analysis of Hox gene expression in the chick limb bud. Development. 1996;122:1449–1466. doi: 10.1242/dev.122.5.1449. [DOI] [PubMed] [Google Scholar]
- Newburger DE, Bulyk ML. UniPROBE: an online database of protein binding microarray data on protein-DNA interactions. Nucleic Acids Res. 2009;37:D77–D82. doi: 10.1093/nar/gkn660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K, Kang J, Subedi KP, Ha JH, Park C. Canine polydactyl mutations with heterogeneous origin in the conserved intronic sequence of LMBR1. Genetics. 2008;179:2163–2172. doi: 10.1534/genetics.108.087114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semerci CN, Demirkan F, Ozdemir M, Biskin E, Akin B, Bagci H, Akarsu NA. Homozygous feature of isolated triphalangeal thumb-preaxial polydactyly linked to 7q36: no phenotypic difference between homozygotes and heterozygotes. Clin Genet. 2009;76:85–90. doi: 10.1111/j.1399-0004.2009.01192.x. [DOI] [PubMed] [Google Scholar]
- VanderMeer JE, Ahituv N. cis-Regulatory Mutations are a Genetic Cause of Human Limb Malformations. Dev Dyn. 2011;240:920–930. doi: 10.1002/dvdy.22535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Ding J, Li YQ, Ren KQ, Sha JH, Zhu MS, Gao X. HnRNP U mediates the long-range regulation of Shh expression during limb development. Hum Mol Genet. 2009;18:3090–3097. doi: 10.1093/hmg/ddp250. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.