Abstract
Neutralizing Abs provide the protective effect of the majority of existing human vaccines. For a prophylactic vaccine against HIV-1, broadly neutralizing Abs (bNAbs) targeting conserved epitopes of the viral envelope glycoproteins (Env) are likely required, as the pool of circulating HIV-1 variants is extremely diverse. The failure to efficiently induce bNAbs by vaccination may be due to the use of sub-optimal immunogens or immunization regimens, or it may indicate that B cells specific for broadly neutralizing Env determinants are selected against during peripheral checkpoints, either before or after antigen encounter. To investigate if perturbation of B cell subsets prior to immunization with recombinant Env protein affects the vaccine-induced Ab response in mice, we used B Lymphocyte Stimulator (BLyS), a cytokine that regulates survival and selection of peripheral B cells. We show that the transient BLyS treatment used here substantially affected naïve B cell populations; in particular, it resulted in an increased number of B cells surviving counter-selection at the transitional stages. We also observed an increased number of mature naïve B cells, especially marginal zone B cells, in BLyS-treated mice. Intriguingly, provision of excess BLyS prior to immunization led to a consistent improvement in the frequency and potency of HIV-1 Env vaccine-induced neutralizing Ab responses, without increasing the number of Env-specific Ab-secreting cells or the Ab binding titers measured after boosting. The results presented here suggest that an increased understanding of BLyS-regulated processes may help the design of vaccine regimens aimed at eliciting improved neutralizing Ab responses against HIV-1.
INTRODUCTION
Efforts to elicit broadly neutralizing Abs (bNAbs) against HIV-1 through envelope glycoprotein (Env) vaccination are so far unsuccessful despite robust antibody (Ab) titers to multiple epitopes on Env stimulated by current vaccine candidates. Even during chronic HIV-1 infection, bNAbs are elicited in only a subset of infected individuals, and usually only after years of active viral replication (1). This suggests that effective B cell responses against bNAb epitopes on Env are infrequent and subject to limitations imposed by extensive immune selection pressure for resistant isolates during infection. The barriers to achieving appropriate Ab specificity and affinity maturation following vaccination are substantial and may be reflective of a variety of factors, including sub-optimal presentation of bNAb epitopes on candidate Env immunogens, insufficient affinity maturation of critical Ab specificities as well as potential limitations in the B cell repertoire caused by events that occur either before or after B cell exposure to antigen.
Developing B cells undergo counter-selection at multiple checkpoints during maturation, resulting in the loss of most emerging BCR reactivities. At the transitional developmental stages (2), about two thirds of newly formed B cells migrating from the bone marrow (BM) die before entering mature pre-immune pools. These losses reflect selection based on BCR signal strength (3, 4) and mediate the elimination of autoreactive and polyreactive specificities in both mice and humans (5, 6). Accordingly, if clonotypes capable of broadly neutralizing activity against HIV-1 are prone to deletion at the transitional stage, their frequency in the pre-immune repertoire may be low to nil. Indeed, some HIV-1 infection-elicited bNAbs share features with specificities prone to elimination during transitional differentiation, such as long heavy chain CDR3 (7) or poly-specificity (8, 9). Alternatively, broadly neutralizing clonotypes or their precursors may survive to populate the pre-immune pools and respond to antigen exposure, yet fail to persist as the immune response evolves and peripheral tolerance mechanisms come into play (10). After antigen activation and co-stimulation, B cells enter the germinal center (GC) reaction where novel specificities are generated through somatic hypermutation (SHM). Among these newly arising specificities, those that most effectively compete for antigen and survival signals selectively persist and differentiate into memory and antibody-secreting plasma cells (11). Thus, if bNAb specificities are rarely generated by SHM, or if these clones are poor competitors within the GC, their entrance into memory or antibody-forming pools may occur at very low frequency.
The B lineage-specific survival factor, BLyS (also termed BAFF), plays key roles in peripheral B cell development, homeostasis, and selection. While BLyS binds three different receptors, its most profound effects are mediated by signaling through BLyS Receptor 3 (BR3, also termed BAFF-R), which is expressed by transitional, mature naïve, and GC B cells (12). There is ample evidence that the BLyS/BR3 axis modulates selection at the transitional stages, since mice and humans deficient in either BLyS or BR3 show severely compromised transitional and mature naïve B cell pools (13, 14), while BLyS over-expression yields B cell hyperplasia and signs of autoimmunity (15, 16). Furthermore, in studies using transgenic mice, exogenous BLyS treatment rescues specificities normally lost at the transitional stage, allowing them to enter the mature pre-immune pools (5). There is evidence for a similar role of the BLyS/BR3 axis in GC evolution (17), although mechanisms have yet to be determined. For example, GCs can be initiated but are not sustained in mice deficient in BLyS (18).
Here, we investigated whether potential counter-selection of bNAb specificities -particularly at the transitional checkpoint - might be overcome by transient manipulation of BLyS levels. We treated mice with BLyS immediately prior to immunization and observed a marked increase in transitional and mature naïve B cell numbers, indicating a significant increase in cells surviving selection at the transitional checkpoint. Upon termination of BLyS treatment, these subsets returned to pre-treatment levels in less than a week. When BLyS- and control-treated mice were immunized with recombinant Env trimers (19), we found a consistent increase in the frequency of animals displaying HIV-1 neutralizing activity in BLyS-treated animals. These results intriguingly suggest that manipulation of selective processes in the peripheral B cell compartment may be used to qualitatively improve vaccine-elicited neutralizing Ab responses against HIV-1.
MATERIALS AND METHODS
Recombinant BLyS and HIV-1 Env glycoproteins
Recombinant human BLyS was kindly provided by Human Genome Sciences, Inc. (Rockville, MD, USA). Recombinant soluble HIV-1 Env gp140 trimers based on the YU2 isolate of HIV-1, gp140-F (19), were produced by transient transfection as previously described (20) and used for immunizations. In brief, cells were transfected at a density of 1.1 × 106/ml in GIBCO®Freestyle293 expression media using 293Fectin, according to manufacturer’s instructions (Invitrogen). Supernatants were collected four days after transfection. Following collection, all supernatants were centrifuged at 3,500g to remove cells or cell debris, filtered through a 0.22mm filter and supplemented with Complete™, EDTA-free protease inhibitor cocktail (Roche) and Penicillin-Streptomycin (Invitrogen). Proteins were captured via glycans by lentil-lectin affinity chromatography (GE Healthcare). After extensive washing with PBS, supplemented with 0.5M NaCl, the proteins were eluted with 1M methyl-α-D-mannopyranoside and captured in the second step via the His-tag by nickel-chelation chromatography (GE Healthcare). Following a wash with 40mM imidazole and 0.5M NaCl in PBS, proteins were eluted with 300mM IM in PBS. Biotinylated Env probes used in the B cell ELISPOT assay (21) were similarly produced.
Treatment of mice and preparation of cells and tissue for analysis
Adult BALB/c mice (Taconic A/S) were injected i.p. with 10μg of recombinant BLyS or PBS once per day for ten days. At indicated time points after treatment, mice were either sacrificed for analysis or immunized s.c. with 10μg of HIV-1 Env in 10μg of the adjuvant AbISCO®-100 (Isconova AB). Animals were boosted once or twice with the same regimen 14 days apart. In some experiments, a third boost was given 60 days after the second boost. Animals were sacrificed at 4 or 21 days after the last immunization. Single cell suspensions were prepared from spleen, BM (two femur and two tibia/mouse) or inguinal lymph node (LN) as described (21). For immunostaining of spleen, tissue was immersed in Optimal Cutting Medium (Histolab) and snap frozen in 2-methyl butane that was kept cold in liquid nitrogen. All animal experiments were approved by the Committee for Animal Ethics (Stockholm, Sweden), and performed according to given guidelines.
Flow cytometry
Single cell suspensions from spleen and LN were stained with following Abs; PerCP anti-B220 (RA3-62B), biotinylated anti-CD23 (B2B4), PE anti-IgM (R6-60.2), PE anti-CD95 (Jo2), APC anti-CD8 (53-6.7) from BD Biosciences, APC anti-CD93 (AA4.1), Alexa Fluor 488 anti-GL7, PE anti-CD1d (1B1), PE anti-CD3 PE (145-2C11) and FITC anti-CD4 (RM4-4) from eBiosciences. Biotinylated antibody was visualized with Alexa Fluor 488-conjugated Streptavidin (Invitrogen). Stained cells were fixed in fixation buffer (BD biosciences) and analyzed on a FACSCalibur (BD Biosciences).
ELISA
The HIV-1 Env-specific ELISA was performed by coating 96-well ELISA plates (Nunc) with 100 μl of soluble HIV-1 Env protein diluted to a concentration of 2 μg/ml in PBS. After overnight (ON) incubation at 4 °C, plates were washed in PBS with 0.05% Tween (wash buffer). The plates were then blocked for 1.5 hrs at RT in PBS with 2% dry milk (blocking buffer). Serum was added in fresh blocking buffer and incubated for 1.5 hrs at room temperature (RT). Plates were washed 6 times in wash buffer and the secondary antibody, goat anti mouse IgG-HRP, was added in wash buffer. Plates were incubated for 1.5 h at RT. The plates were washed 6 times in wash buffer and the assay was developed using the SigmaFAST OPD kit (Sigma-Aldrich). The reaction was stopped by the addition of 1M H2SO4 and the OD was read at 492 nm using an Asys Expert 96 ELISA reader (Biochrom).
For detection of anti-dsDNA Abs, ELISA plates were pre-coated with 50 μl of met-BSA (Sigma) diluted to a concentration of 5 μg/ml in PBS and incubated for 6 hrs at 4°C. Plates were then washed 4 times in PBS with 0.05 % Tween (wash buffer), and coated with 50 μl DNA (Sigma) diluted to a concentration of 50 μg/ml in PBS. For detection of anti-PC Abs, ELISA plates were coated with 50 μl of PC-BSA diluted to a concentration of 2.5 μg/ml in PBS at 4°C ON. After washing 4 times in wash buffer, both sets of plates were blocked with PBS containing 1.5 % BSA, 0.1 % gelatine and 3mM EDTA (blocking buffer) for 2 hrs at RT. Serum was then added in fresh blocking buffer and incubated for 2 hrs at RT prior to washing 4 times in wash buffer. Secondary Abs, anti-mouse IgM-ALP (Mabtech) or anti-mouse IgM-HRP (Southern Biotech), were added in blocking buffer and incubated for 1 hr at RT. Depending on the secondary Ab used, the ELISA was developed using either the SIGMA FAST p-Nitrophenyl phosphate mix (SIGMA) and the reaction was stopped by adding 3 N NaOH, or using the SureBlue TMB Microwell Peroxidase Substrate (KPL) and the reaction was stopped by adding TMB Stop Solution (KPL). The OD was measured at 405 or 450 nm respectivelyusing an Asys Expert 96 ELISA reader (Biochrom).
The cardiolipin ELISA was performed by coating 96-well ELISA plates (Nunc) with 50 μl of cardiolipin (SIGMA) diluted to a concentration of 50 μg/ml in 99.5% EtOH. The plates were allowed to evaporate completely in 4 °C ON. The plates were blocked in PBS with 10% FCS (blocking buffer) for 1 hr at 37 °C. Serum was added in fresh blocking buffer and incubated for 1 hr at 37 °C. The plates were then washed in PBS only. Secondary antibody anti-mouse IgM-HRP (Southern biotech) was added in blocking buffer and plates were incubated for 1 hr at 37 °C. Plates were washed in PBS only and developed as described above.
To measure total IgM levels in serum, 96-well ELISA plates (Nunc) were coated with 100 μl of polyclonal goat anti-mouse IgM (Southern Biotech) diluted to a concentration of 2 μg/ml in 0.05 M Carbonate buffer. Plates were incubated ON at 4 °C. Next. ELISA plates were washed 6 times in PBS with 0.05% Tween (wash buffer) and blocked for 1.5 hrs in PBS with 5% FCS (blocking buffer) at RT. Serum was added to fresh blocking buffer and incubated for 2 hrs at RT. The plates were washed 6 times in wash buffer and secondary antibody, anti-mouse IgM-ALP (Mabtech) or anti-mouse IgM-HRP, was added diluted in blocking buffer. After 1.5 hrs of incubation at RT the plates were washed 6 times in wash buffer. The assay was developed as described above.
B cell ELISpot
Env-specific and total IgG ASC were enumerated in a B cell ELISpot assay as previously described (21). Briefly, 96-well MultiScreen-IP filter plates (Millipore) were pre-treated with 70% ethanol and washed 3 times in sterile PBS, before coated with 1μg/well (10μg/ml) of a polyclonal goat anti-mouse IgG antibody (Mabtech AB). The plates were incubated over night at 4 °C. 2 h before addition of the cells, the plates were washed 5 times in sterile PBS and blocked with complete RPMI medium at 37 °C for 2 h. Cells were added in duplicates to the wells in 3-fold serial dilutions, starting at 106 cells/well. For in vitro stimulation of memory B cells, splenocytes were cultured for six days with or without 2μg/ml LPS. Plates were then wrapped in plastic wrap and incubated for 12 h at 37 °C. For detection of spots, the cells were removed by washing the plates 6 times in PBS with 0.05% Tween-20. Total IgG secreting cells were detected with 100ng/well (1μg/ml) of a biotinylated polyclonal goat anti-mouse IgG (Mabtech AB) in blocking buffer (PBS with 1% FCS and 0.05% Tween 20). For the detection of Env-specific B cells, 200ng/well (2μg/ml) of biotinylated protein (variants of Env or β-gal as a control) was added diluted in blocking buffer. Biotinylated antibody and protein were incubated in the plates for 2 h at RT. Plates were then washed 6 times in PBS before 100μl of ALP-conjugated streptavidin (Mabtech AB) diluted 1:1000 in PBS was added. Plates were incubated for 45 min at RT and then washed 6 times in water. 100μl of BCIP/NBT-plus substrate (Mabtech AB) was then added and incubated for approximately 10 min at RT. Plates were washed extensively with water and air-dried. Spots were counted in an ImmunoSpot® analyzer (Cellular Technology Ltd.). Memory b cell-derived ASC were calculated by subtracting the background of non LPS-stimulated from LPS-stimulated cultures.
Immunohistology and confocal microscopy
Spleens were frozen in OCT medium and cryo-sectioned (6–8μm) onto Superfrost Ultra Plus microscope slides (VWR). Staining was performed with the following Abs; APC anti-B220 (RA3-6B2) from eBiosciences, biotinylated MOMA-1 from abcam and Alexa Fluor 488 anti-TcRβ (H57-597) from BioLegend. Biotinylated Ab was visualized with Cy3-conjugated Streptavidin from Jackson Immunoresearch. Slides were scanned on a Leica TCS SP5X, using a 10X/0.30 magnification objective: Leica 506505 HCX FLUOTAR. The Leica Application Suite Advanced Fluorescence software was used to prepare images and ImageJ was used for final adjustment of brightness and contrast.
Neutralization assays
Neutralization assays were performed using single round of infection HIV-1 Env pseudoviruses and TZM-bl target cells as previously described (22, 23). In total, we had enough sera from individual mice to analyze neutralization against four Tier 1 viruses (MN, HXBc2, SF162 and BaL) and one Tier 2 virus (6535). Results are reported as the serum neutralization ID50, the reciprocal of the serum dilution producing 50% virus neutralization without subtracting pre-bleed values. For competition neutralization assays, test or control ligand was added to the serum 30 minutes prior to the addition of virus. V3-specific activity was mapped using a YU2-derived V3 peptide and a scrambled control peptide as previously described (24). CD4 binding site (CD4bs)-directed neutralizing activity was mapped using a pair of probes referred to as TriMut and TriMut368/70 (25, 26).
Statistical analysis
Data that passed the D’agostino and Pearson omnibus normality test were analyzed by unpaired, two-tailed, Student’s t-tests, and data that did not pass by the Mann-Whitney test. GraphPad Prism software was used for all analyses and data were considered significant at * for p ≤ 0.05, ** for p ≤ 0.01 and *** for p ≤ 0.001.
RESULTS
Exogenous BLyS treatment increases primary B cell numbers but does not alter splenic architecture
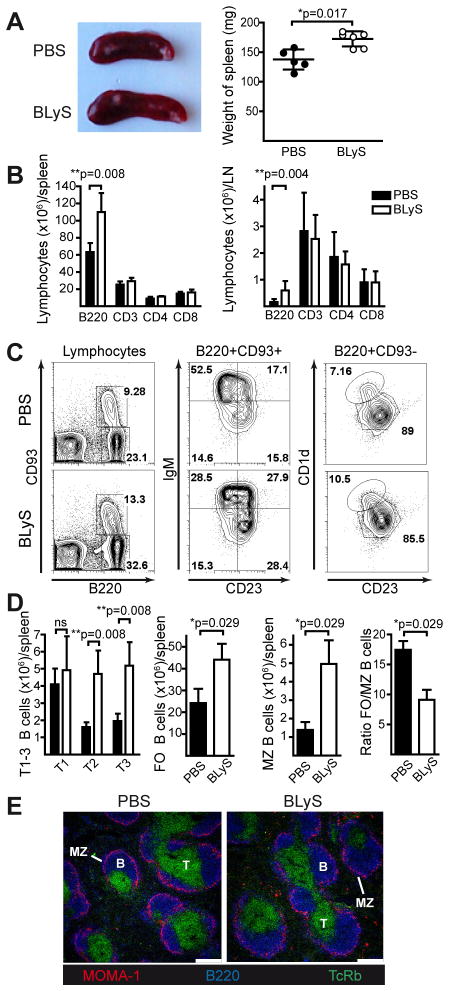
To characterize the effect of transient BLyS treatment on B cell compartments, we injected mice with recombinant BLyS or PBS (controls) daily for ten days prior to analysis. Consistent with prior reports (27), spleen size and weight increased in BLyS-treated mice (Fig. 1A), reflecting expanded B cell numbers without significant effects on T cells (Fig. 1B, left panel). A similar effect on B cell populations was seen in LN (Fig. 1B, right panel). The numbers of transitional (T2 and T3) B cells increased significantly in BLyS-treated mice and there was an accumulation of both follicular (FO) and marginal zone (MZ) B cells, especially MZ B cells as shown by the decreased FO/MZ B cell ratio (gated populations are shown in Fig. 1C, calculated as absolute numbers in Fig. 1D). Immunofluorescence analysis of frozen spleen sections from PBS- and BLyS-treated mice showed normal splenic architecture suggesting that trafficking and homing of cells were largely unaffected by BLyS treatment (Fig. 1E).
Figure 1. BLyS treatment increases B cell numbers but leaves the splenic architecture unaffected.
Mice were injected with PBS or BLyS once per day for 10 days and sacrificed on day 11 for analysis of lymphocytes in spleen and LN. (A) Representative image of spleens (left panel) and measurements of spleen weight (right panel). (B) Effect of BLyS treatment on B (B220+) and T cell (CD3+, CD3+CD4+ and CD3+CD8+) numbers in spleen (left panel) and the two inguinal LNs (right panel). (C) Representative flow cytometry plots of B cell subsets in PBS- and BLyS-treated mice; left panel: immature naïve B cells (B220+, CD93+) and mature naïve B cells (B220+, CD93−), middle panel: T1 (IgMhi, CD23−), T2 (IgMhi, CD23+) and T3 (IgMlo, CD23+) and right panel: FO (CD23hi, CD1dlo) and MZ B cells (CD23low, CD1dhi). (D) Effect of BLyS treatment on splenic B cell subsets: transitional (T1, T2, T3), follicular (FO), and marginal zone (MZ), and ratio of FO/MZ B cells in PBS-and BLyS-treated mice. Black bars: PBS-treated, white bars; BLyS-treated. (E) Confocal microscopy images of spleen sections; TCRβ (T cells, green), B220 (B cells, blue) and metallophilic macrophages (red). The MZ area is present outside of metallophilic macrophages as indicated. White bar represents 250μm. Mean values ±SD is shown in all diagrams. Data is representative of two independent experiments with 5–6 mice/group.
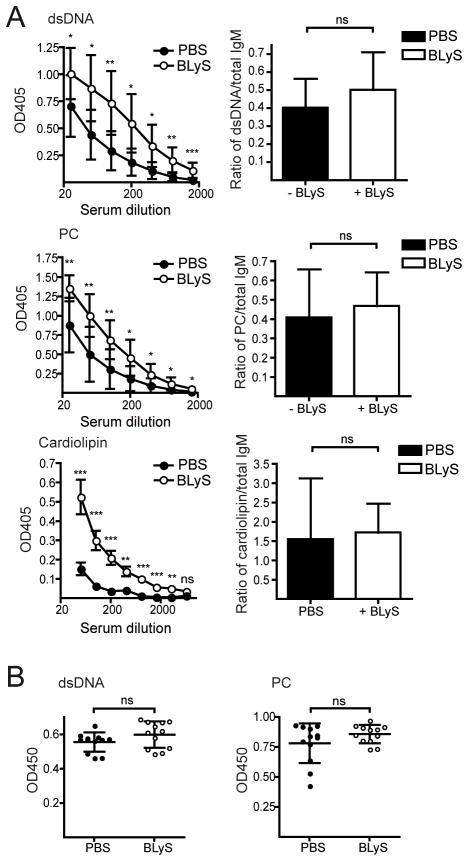
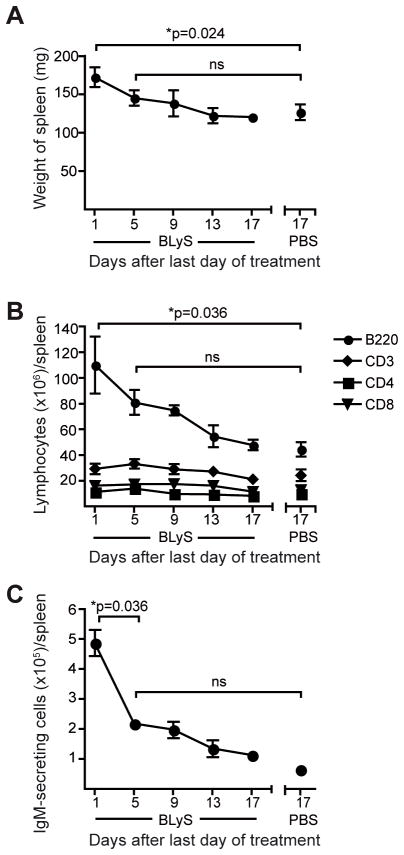
Under conditions where the BLyS level is permanently increased and the homeostatic regulation of B cells is defective, such as in BLyS transgenic mice, immature self-reactive B cells survive peripheral selection checkpoints and progress to mature naïve pools (5, 15, 16, 28, 29). To investigate whether BLyS treatment promoted survival of autoreactive B cells, we measured Ab titers against dsDNA, phosphorylcholine (PC) and cardiolipin. Immediately after cessation of BLyS treatment, we found elevated levels of both anti-dsDNA, anti-PC and anti-cardiolipin Abs (Fig. 2A, left panels). However, the total Ab levels at this time point were also increased and when normalized to these there was no difference between BLyS-and control-treated animals (Fig. 2A, right panels). Furthermore, when anti-dsDNA and anti-PC Ab responses were measured 19 days after termination of BLyS-treatment, the levels were no longer increased in the BLyS-treated mice (Fig. 2B). Within 5 days of the last BLyS treatment, spleen weights (Fig. 3A), B lymphocyte numbers (Fig. 3B) and the number of IgM-secreting cells per spleen (Fig. 3C) also approached pre-treatment levels. Accordingly, we chose to initiate Env immunizations one day after the last day of BLyS treatment, when peripheral B cells were maximally affected.
Figure 2. Effect of BLyS treatment on anti-dsDNA, anti-PC and anti-cardiolipin Ab levels in serum.
(A) IgM against dsDNA (top left panel), PC (middle left panel) and cardiolipin (lower left panel), in sera collected 1 day after the last day of PBS- or BLyS treatment. Ratio of anti-dsDNA IgM (at serum dilution 1:100), anti-PC IgM (at serum dilution 1:100) and anti-cardiolipin (at serum dilution 1:50) against total IgM (at serum dilution 1:4050) in serum at 1 day after the last day of PBS- or BLyS treatment (right panels). (B) IgM against dsDNA (left panel) or PC (right panel), in sera collected 19 days after the last day of PBS- or BLyS treatment. OD450 values at a serum dilution of 1:25 are shown. In (A) pooled data from two individual experiments are shown with a total of 9–10 mice/group. In (B), data from one experiment with 11–12 mice/group is shown. Diagrams show mean values ±SD. Statistical difference are shown as * for p-values ≤ 0.05, ** for p-values ≤ 0.01 and *** for p-values ≤ 0.001.
Figure 3. The effect of BLyS treatment on lymphocyte populations is transient.
At different time points after day 10 of BLyS or PBS treatment, (A) the weight of the spleen, (B) flow cytometric analysis showing the total numbers of B220+, CD3+, CD4+ and CD8+ cells in spleen and (C) B cell ELISpot analysis of the number of total IgM-secreting cells in spleen were measured. Mean values ±SD are shown in all diagrams. The data are from one experiment with 3–6 mice per/group.
BLyS treatment prior to Env immunization does not affect the magnitude of the Env-specific B cell response
Co-delivery of antigen fused to BLyS or the use of BLyS as an adjuvant component administered at the time as the antigen has previously been reported (30–32). Here, we investigate the magnitude and quality of Env vaccine-elicited B cell responses after BLyS pre-treatment, using HIV-1 gp140-F trimers in adjuvant. The gp140-F trimers possess a heterologous trimerization motif (F) appended to the Env ectodomain (gp140) and are derived from the primary HIV-1 isolate, YU2 (19) (Fig. 4A). Immunized mice were sacrificed for analysis 4 or 21 days after the boost and the presence of mature splenic B cells with a GC phenotype was investigated. We detected no significant difference in the number of cells with this phenotype between BLyS- and control treated mice at either time point after the boost, suggesting that GC formation is not sensitive to BLyS levels (Fig. 4B), consistent with studies in BLyS-deficient mice (18). Furthermore, quantification of the number of antibody-secreting cells (ASC) by B cell ELISpot analyses of splenocytes collected four days after the boost showed similar levels of total IgG- and Env-specific IgG-secreting cells in BLyS- and control-treated mice (Fig. 4C, left panel).
Figure 4. Germinal centers and Env-specific B cell numbers are similar in PBS- and BLyS-treated mice.
(A) Schematic of the gp140-F Env immunogen and the treatment/immunization regimen. (B) Representative flow cytometry plots showing splenic GC B cells (B220+, CD93−, GL7hi and CD95hi) in PBS-treated (upper panels) and BLyS-treated mice (lower panels) 21 days after boosting. Right panel shows splenic GC cells in animals terminated at 4 or 21 days after boosting. (C–E) Enumeration of Env-specific B cells by B cell ELISpot analysis; total IgG- and gp140-specific cells (left panels) and sub-region-specific cells (gp41, V123 and Other, right panels); (C) ASC in spleen 4 days after boost, (D) ASC in BM 21 days after boost and (E) ASC from LPS-cultured splenocytes collected 21 days after boost. Mean values ±SD are shown in all diagrams. The data in B (4 days) are representative of two independent experiments with 7–12 mice/group and in C (left panel) of three independent experiments with 4–12 mice/group. The remaining results in B–E are from one experiment with 12 mice/group.
We next asked if the response directed against different sub-regions of Env differed between BLyS-treated mice and controls. This analysis was performed using a differential B cell ELISpot assay that allows the enumeration of B cells specific for distinct sub-determinants of gp140-F, including gp41, variable region 1, 2 and 3 (V123), and non-gp41, non-V123 reactivities not clearly defined and referred to as “Other” (21). We found that a substantial fraction of the response after the boost was directed against gp41, consistent with previous results (21), with no difference between the two groups of mice (Fig. 4C, right panel). On day 21 post boost, a pool of Env-specific plasma cells is expected to have accumulated in the BM. When this population was analyzed, we detected no marked differences between BLyS-treated mice and controls in the number of IgG-secreting cells against total Env or Env sub-determinants, except an increase in the non-gp41, non-V123 fraction referred to as “Other” in BLyS-treated animals. However, this difference was not consistently observed (Fig. 4D). We also examined the Env-specific B cell memory compartment after in vitro LPS stimulation of splenocytes collected 21 days after the second immunization and observed an increase in the number of memory B cells in BLyS-treated animals compared to controls, but no difference in the number of Env-specific or Env subspecific memory B cells (Fig. 4E). Taken together, these data demonstrate that BLyS treatment prior to immunization did not affect the magnitude of the total Env-specific B cell response.
BLyS treatment prior to immunization increases the frequency and potency of neutralizing Ab responses against a subset of viruses
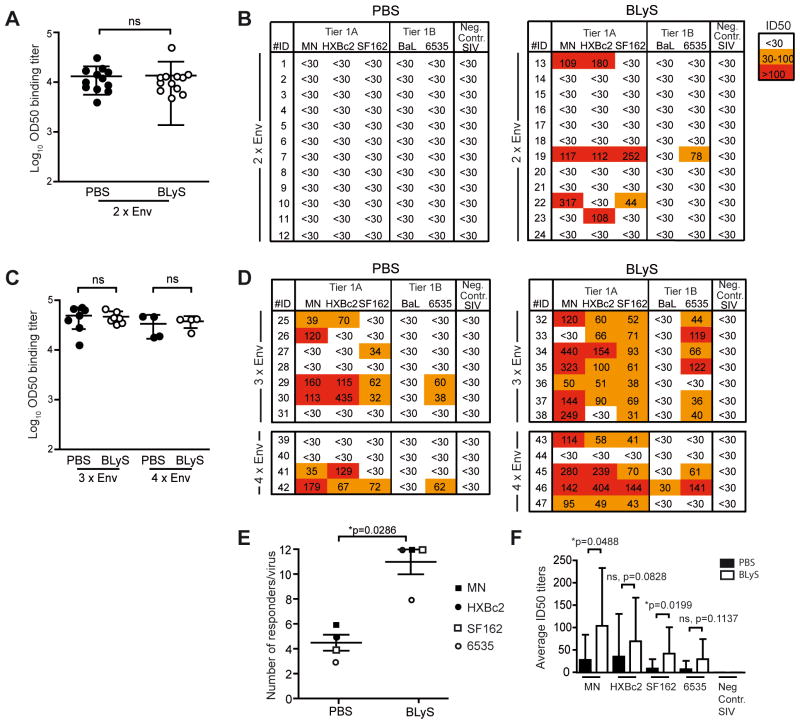
To investigate the quality of the elicited B cell response at a functional level, we next focused on the serological Ab response. We first assessed Ab binding titers against YU2 Env in BLyS- and control-treated mice immunized twice with the HIV-1 Env trimers. Despite the profound difference in B cell subsets observed at the time of priming, we detected similar titers of Env-specific Abs in the two groups (Fig. 5A) in agreement with the B cell ELISpot results. Next, we sought to examine the capacity of the sera to neutralize diverse HIV-1 strains, a powerful assay to detect qualitative or quantitative differences in elicited Ab functional activity. We used a panel of five heterologous Tier 1A and 1B HIV-1 Env pseudotyped viruses and a negative control virus pseudotyped with SIV Env, which was the maximum number of viruses we could include based on the available serum volumes. When these viruses were used in a well-standardized neutralization assay (22), we detected neutralizing activity against the Tier 1A clade B viruses (MN, HXBc2 and SF162) and the clade B Tier 1B virus (6535) in sera from some BLyS-treated animals while this was not observed in control-treated mice (Fig. 5B). To confirm this effect, we performed two independent immunization experiments with additional boosts (3xEnv and 4xEnv), but otherwise replicated the same conditions used in the first experiment (2xEnv). Overall, the frequency of mice displaying detectable neutralization titers was increased in these repeat experiments compared to the first experiment, likely reflecting higher Env-binding titers elicited by the additional boosts (Fig. 5C). Consistent with the first experiment, we saw no difference in total Env-binding Ab titers between BLyS- and control treated animals, yet the neutralizing activity against MN, HXBc2, SF162 and 6335 was more frequent and more potent in BLyS-treated animals with almost all mice displaying neutralizing activity, some at relatively high titers (Fig. 5D).
Figure 5. BLyS treatment prior to immunization increases the serum neutralizing Ab responses.
(A) Sera from mice immunized twice (2x) with Env in adjuvant collected 21 days after the second immunization were analyzed for total Env-binding Abs (left panel) and for neutralizing activity against a panel of heterologous HIV-1 isolates (right panel). OD50 values are shown for total Env-specific Ab titers. Neutralization data are shown as the reciprocal dilution giving 50% neutralization (ID50 titer). (B) The same analysis as in (A) using sera from animals immunized three (3x) or four times (4x) with Env in adjuvant collected 4 days after the last immunization. Each point in the ELISA analysis represents data from one mouse and the bar shown in (B) indicates the mean of all values ±SD. (C) The frequency of PBS- and BLyS treated mice with neutralization activity against MN, HXBc2, SF162 or 6535, combining all experiments (2xEnv, 3xEnv and 4xEnv), were plotted and analyzed by a non-parametric Mann-Whitney test. To have an equal number of mice in each group one mouse was removed from the 4xEnv BLyS group. This mouse (number 46) displayed the highest ID50 neutralization titer against several of the viruses. Thus, by removing this mouse from the comparison we under-estimate rather than over-estimate the effect caused by the BLyS treatment. (D) The potency of neutralization was also analyzed by calculating the average ID50 neutralization titers against MN, HXBc2, SF162 and 6535 among PBS- and BLyS-treated mice, combining the three experiments (2xEnv, 3xEnv and 4xEnv). Statistical significance was analyzed using the non-parametric Mann-Whitney test. The ELISA data from 2x are from one experiment with 12 mice/group; the results from 3x are representative data from two independent experiments with 5–7 mice/group, and the data from 4x are from one experiment with 4–5 mice/group. The neutralization data are from one experiment each for 2x (12 mice/group), 3x (7 mice/group) and 4x (4–5 mice/group).
When the number of mice responding with positive neutralizing activity against MN, HXBc2, SF162 and 6335 in the control- and BLyS-treated groups was compared, we observed a statistically significant difference (Fig. 5E). We also observed statistically significant differences between the PBS and BLyS-treated mice when the average ID50 neutralization titers against MN and SF162 were compared, but not against HXBc2 and 6535 (Fig. 5F). There was no correlation between YU2 Env binding titers and virus neutralizing activity (Fig. S1A), suggesting that the improved neutralizing activity of the serum Abs was not simply due to an increase in overall Ab titers against the antigen used for immunization. Since YU2 Env is heterologous to the viruses used in the neutralization assay, we also measured serum binding titers against recombinant MN and SF162 Env proteins. These analyses showed increased binding titers against MN, providing a possible explanation for the improved MN neutralizing activity in BLyS-treated animals. However, a similar relationship between binding and neutralization was not observed for SF162 (Fig. S1B).
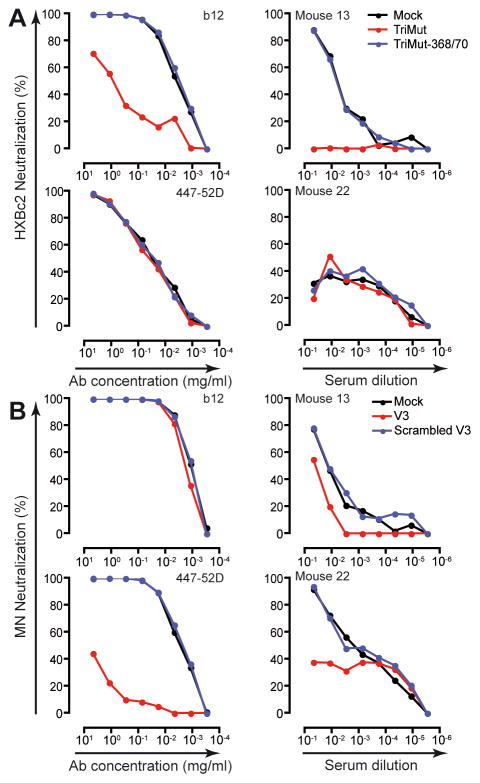
The specificity of the HIV-1 neutralizing activity was confirmed by two independent assays, the first of which was to use SIV Env, which is not cross-reactive with HIV-1 Env, resulting in no activity above background in any of the sera (Fig. 5B and D). To further confirm the specificity of the neutralizing activity elicited in BLyS-treated mice, and to map the response to distinct antigenic regions on Env by ligand competition analysis, we tested selected sera from BLyS-treated mice displaying the highest neutralization titers. The ligands included in these analyses were designed to differentially detect neutralizing Ab activity directed against the CD4 binding site (CD4bs), TriMut and TriMut-368/70, described in (26), and the V3 region (V3 peptide and scrambled V3 peptide). To validate this mapping approach, we show that neutralization of HXBc2 by the CD4bs-directed monoclonal Ab IgGb12 was effectively absorbed by TriMut but not by TriMut-368/370, while no neutralization of HXBc2 was detected with the V3-directed monoclonal Ab 447-52D (Fig. 6A, left panels). Neutralization of MN by 447-52D was absorbed by the V3 peptide but not by the scrambled V3 control peptide and no neutralization was detected of MN with IgGb12 (Fig. 6B, left panels). When analyzing the sera from BLyS-treated mice, we found that CD4bs-directed Abs were responsible for a significant fraction of the neutralizing activity against HXBc2 in mouse #13 (Fig. 6A, upper right panel), while V3-specific Abs were responsible for a significant fraction of the neutralizing activity against MN in mouse #22 and part of the neutralizing activity in mouse #13 (Fig. 6B, right panels). These analyses confirm that the Ab response in these responding mice was antigen-specific and directed against distinct neutralizing determinants of HIV-1 Env.
Figure 6. Distinct Env-reactivities are responsible for the neutralizing activity in serum of BLyS-treated mice.
To map the neutralizing activity against selected viruses, sera from responding BLyS-treated mice were selected and analyzed by ligand competition analysis using pairs of probes designed to detect CD4bs-directed (TriMut and TriMut-368/70) or V3-directed (V3 and a scrambled V3 peptide) neutralizing Ab activity. The probes were individually pre-incubated with serial diluted control Ab or sera from BLyS-treated mice immunized twice with Env in adjuvant prior to adding the virus. Differential neutralization curves of Abs and sera are shown as percent neutralization against (A) HXBc2 and (B) MN.
DISCUSSION
Current knowledge suggests that HIV-1 has evolved to render conserved Env determinants poorly immunogenic in order to escape recognition by bNAbs. Consistent with this, it has been suggested that some bNAb epitopes on Env display self-like properties and necessitate auto-reactive antibodies for efficient epitope recognition (8, 10, 33). Such regions include the membrane proximal external region (MPER) of gp41, recognized by the prototypic MPER-directed MAbs 2F5 and 4E10, which were shown to cross-react with cardiolipin (8). While the role of cardiolipin cross-reactivity and the relationship of that property in regards to efficient virus neutralization has been a subject of some debate (34), it was shown that 4E10 depends on the presence of aromatic amino acids in its heavy chain CDR3 region for efficient recognition of its epitope, perhaps through lipophilic interactions (35). Furthermore, poly-reactivity appears to be a common feature of neutralizing antibodies elicited during chronic HIV-1 infection (9, 36, 37). Thus, the possibility exists that B cells specific for some bNAb targets are subject to counter-selection at one or several stages during the path towards an antibody-secreting cell. It follows that if such processes are at play, they may diminish the likelihood of eliciting desired neutralizing B cell responses by vaccination. To investigate this possibility experimentally, we established a system to perturb B cell homeostasis and selection by transient provision of recombinant BLyS, prior to immunization with HIV-1 Env. We show that the BLyS treatment markedly increased the proportion of B cells surviving through the transitional stages resulting in substantially increased numbers of follicular and MZ B cells and a reduced ratio of follicular to MZ B cells, consistent with previous reports (38). The effect of BLyS was short-lived, with B cell numbers returning to near baseline levels already five days after treatment termination.
When mice were primed with soluble Env trimers in adjuvant, on one day after termination of the BLyS treatment when the B cell populations were maximally affected, a substantial and consistent increase in the frequency of mice displaying neutralizing activity was observed in the BLyS-treated group at the time of serum analysis, 18 days or more after priming. We did not observe an improvement in terms of breadth of neutralization, but neutralizing activity in the BLyS-treated animals was more frequent and, against some viruses, more potent and could be assigned to distinct Env sub-reactivities by serological mapping analysis of samples with high neutralizing titers. Interestingly, the frequency of Env-specific antibody-secreting cells as measured by B cell ELISpot analysis in multiple B cell compartments, including splenic plasma cells (Fig. 4C), bone marrow plasma cells (Fig. 4D) and in vitro cultured splenic memory B cells (Fig. 4E) after boosting were comparable between control and BLyS-treated mice. We also did not observe a difference between the groups in terms of the total binding antibody titers to the YU2 Env immunogen, suggesting that despite a larger naïve B cell pool at the time of priming the magnitude of the vaccine-induced response was not augmented. This may be consistent with effective feed-back mechanisms, such as FCRγII signaling, regulating the magnitude of the elicited B cell response. When the relationship between YU2 Env binding and neutralizing activity was analyzed for individual mice, we observed no correlation for any of the viruses against which neutralizing activity was detected (r2=0.20 for MN, r2=0.21 for HXBc2, r2=0.040 for SF162 and r2=0.085 for 6435) (Fig. S1A). Instead, several mice displaying high Env binding titers did not possess detectable neutralizing activity and conversely, neutralizing activity was occasionally detected in serum displaying modest Env binding titers. When binding titers were measured against the matched Env glycoprotein, improved neutralization of MN could be explained by an increased binding titer to the homologous (MN) Env in some mice. However, a similar effect was not observed for SF162-directed neutralization as there were no difference between BLyS-treated and control mice in terms of binding antibodies against SF162 Env, and the individual mice in the BLyS group displaying neutralizing activity against SF162 (#19 and #22) were not the same as those with the highest binding titers to SF162 Env (Fig S1B). Collectively, this analysis suggests that the BLyS pre-treatment had a qualitative effect on the Env-specific B cell response.
There are several possible explanations for the observed effects as BLyS can affect B cell survival at multiple stages. The best-established activity of BLyS is at the transitional checkpoint where, under normal circumstances, immature B cells compete for available BLyS before surviving clones mature and populate the B cell follicles (14, 39). Recent evidence suggests that BLyS also modulates germinal center evolution (reviewed in Kalled et al. (40)). In the studies presented here, we believe that it is most likely that BLyS improved survival of B cells prior to BCR-specific activation, thereby affording increased representation of antigen-responsive neutralizing clonotypes in BLyS-treated mice. We consider it less likely that the effect arose from BLyS modulating the GC reaction since we only administered BLyS prior to the first Env immunization, and the effects of the BLyS treatment we measured were very short-lived. In fact, we limited the time frame during which exogenous BLyS was given in order to minimize the possibility that the exogenous BLyS would affect the fate of B cells after they had encountered antigen. Thus, the experimental conditions used here were chosen to modulate the B cell pools prior to immunization rather than to affect processes at later time points. This is a distinctly different approach from that used in a recent study by Melchers et al. where BLyS (BAFF) was co-administered with an HIV-1 Env antigen through plasmid DNA vaccination (31), thus the effect of BLyS in that system is expected to be different from the experimental set-up described here.
We have not shown directly that naïve B cells capable of producing neutralizing Abs were rescued by the exogenous BLyS treatment and responded to the vaccination. However, our cellular analyses strongly suggest that excess BLyS levels substantially affected the composition of transitional and mature naïve B cell pools, consistent with an altered naïve repertoire. This is reflected by the disproportionate enlargement of the marginal zone (MZ) pool, and the shift in the transitional B cells to a greater proportion and number of later transitional subsets (T2 and T3 cells; CD23+ CD93+) in BLyS-treated mice compared with PBS-treated mice. While we have not analyzed the B cell repertoire at the molecular level, the MZ is well established as a pool that contains a different repertoire of B cells (41, 42). Furthermore, an enlargement of the late TR pool indicates a relaxation of negative selection that normally occurs at this checkpoint (5, 28, 43, 44). Thus, our cellular analysis strongly supports the statement that we have altered the conditions under which the Env-specific B cell response is elicited, which is reflected in a higher frequency of mice responding with detectable neutralizing activity and in several cases higher neutralizing titers (Fig 4). A molecular characterization of the pre-immune B cell repertoire in BLyS-treated mice will help to elucidate this question and is the focus of our future efforts. Similarly, an analysis of the Env-specific B cell repertoire in control and BLyS-treated mice is needed to fully dissect the effects observed here.
It is interesting to note that increased levels of transitional B cells were reported in individuals chronically infected with HIV-1 (45). Along with CD4+ helper T cell destruction caused by the infection, HIV-1 infected individuals often present multiple alterations in peripheral B cell subsets, including decreased stringency at peripheral B cell selection checkpoints, which may be a common consequence of chronic immune activation (46). The observation that a subset of chronically infected individuals develop potent and broad serum neutralizing activity after several years of infection (47–49) suggests that the human immune system is capable of generating such Ab responses. Consistent with this, bNAbs elicited by natural infection display extensive SHM, and when back-mutated to their germ-line sequence lose broadly neutralizing activity (49). Thus, activation of rare B cell clones and successive rounds of GC selection to enhance otherwise poorly competitive specificities, may be required before broadly neutralizing activity is achieved.
In summary, we established a system that can be used to improve our understanding of HIV-1 Env vaccine-induced B cell responses. We show that manipulation of naïve B cell subsets by exogenous BLyS treatment prior to vaccination with recombinant Env trimers in adjuvant resulted in an improvement in the elicited HIV-1-specific neutralizing antibody response. These studies provide a foundation for mechanistic investigations of BLyS-regulated peripheral B cell selection processes and their roles for vaccine-induced neutralizing Ab responses.
Supplementary Material
Acknowledgments
We are grateful to T-S. Migone and Human Genome Sciences for recombinant BLyS, M. Forsell, K. Sandgren and R. Goenka for scientific discussions and assistance with microscopy and to the personnel at the MTC animal facility at Karolinska Institutet for excellent technical assistance.
Abbreviations used in the manuscript
- Env
envelope glycoprotein
- BLyS
B Lymphocyte Stimulator
- bNAb
broadly neutralizing Ab
- BM
bone marrow
- GC
germinal center
- SHM
somatic hypermutation
- BR3
BLyS Receptor 3
- LN
lymph node
- RT
room temperature
- MZ
marginal zone
- FO
follicular
Footnotes
This study was supported by the Swedish Research Council, Sida/SAREC, Karolinska Institutet (PD and GKH), the International AIDS Vaccine Initiative (GKH and RTW) and the National Institute of Allergy and Infectious Diseases at the National Institutes of Health for intramural funding to the Vaccine Research Center (JRM) and grant AI073939S1 (MPC).
References
- 1.Stamatatos L, Morris L, Burton DR, Mascola JR. Neutralizing antibodies generated during natural HIV-1 infection: good news for an HIV-1 vaccine? Nat Med. 2009;15:866–870. doi: 10.1038/nm.1949. [DOI] [PubMed] [Google Scholar]
- 2.Allman D, Lindsley RC, DeMuth W, Rudd K, Shinton SA, Hardy RR. Resolution of three nonproliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. J Immunol. 2001;167:6834–6840. doi: 10.4049/jimmunol.167.12.6834. [DOI] [PubMed] [Google Scholar]
- 3.Gu H, Tarlinton D, Muller W, Rajewsky K, Forster I. Most peripheral B cells in mice are ligand selected. J Exp Med. 1991;173:1357–1371. doi: 10.1084/jem.173.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levine MH, Haberman AM, Sant’Angelo DB, Hannum LG, Cancro MP, Janeway CA, Jr, Shlomchik MJ. A B-cell receptor-specific selection step governs immature to mature B cell differentiation. Proc Natl Acad Sci U S A. 2000;97:2743–2748. doi: 10.1073/pnas.050552997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thien M, Phan TG, Gardam S, Amesbury M, Basten A, Mackay F, Brink R. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity. 2004;20:785–798. doi: 10.1016/j.immuni.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 7.Saphire EO, Parren PW, Pantophlet R, Zwick MB, Morris GM, Rudd PM, Dwek RA, Stanfield RL, Burton DR, Wilson IA. Crystal structure of a neutralizing human IGG against HIV-1: a template for vaccine design. Science. 2001;293:1155–1159. doi: 10.1126/science.1061692. [DOI] [PubMed] [Google Scholar]
- 8.Haynes BF, Fleming J, St Clair EW, Katinger H, Stiegler G, Kunert R, Robinson J, Scearce RM, Plonk K, Staats HF, Ortel TL, Liao HX, Alam SM. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science. 2005;308:1906–1908. doi: 10.1126/science.1111781. [DOI] [PubMed] [Google Scholar]
- 9.Mouquet H, Scheid JF, Zoller MJ, Krogsgaard M, Ott RG, Shukair S, Artyomov MN, Pietzsch J, Connors M, Pereyra F, Walker BD, Ho DD, Wilson PC, Seaman MS, Eisen HN, Chakraborty AK, Hope TJ, Ravetch JV, Wardemann H, Nussenzweig MC. Polyreactivity increases the apparent affinity of anti-HIV antibodies by heteroligation. Nature. 2010;467:591–595. doi: 10.1038/nature09385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verkoczy L, Diaz M, Holl TM, Ouyang YB, Bouton-Verville H, Alam SM, Liao HX, Kelsoe G, Haynes BF. Autoreactivity in an HIV-1 broadly reactive neutralizing antibody variable region heavy chain induces immunologic tolerance. Proc Natl Acad Sci U S A. 2010;107:181–186. doi: 10.1073/pnas.0912914107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gatto D, Brink R. The germinal center reaction. J Allergy Clin Immunol. 2010;126:898–907. doi: 10.1016/j.jaci.2010.09.007. quiz 908–899. [DOI] [PubMed] [Google Scholar]
- 12.Stadanlick JE, Kaileh M, Karnell FG, Scholz JL, Miller JP, Quinn WJ, 3rd, Brezski RJ, Treml LS, Jordan KA, Monroe JG, Sen R, Cancro MP. Tonic B cell antigen receptor signals supply an NF-kappaB substrate for prosurvival BLyS signaling. Nat Immunol. 2008;9:1379–1387. doi: 10.1038/ni.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gross JA, Dillon SR, Mudri S, Johnston J, Littau A, Roque R, Rixon M, Schou O, Foley KP, Haugen H, McMillen S, Waggie K, Schreckhise RW, Shoemaker K, Vu T, Moore M, Grossman A, Clegg CH. TACI-Ig neutralizes molecules critical for B cell development and autoimmune disease. impaired B cell maturation in mice lacking BLyS. Immunity. 2001;15:289–302. doi: 10.1016/s1074-7613(01)00183-2. [DOI] [PubMed] [Google Scholar]
- 14.Schiemann B, Gommerman JL, Vora K, Cachero TG, Shulga-Morskaya S, Dobles M, Frew E, Scott ML. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293:2111–2114. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- 15.Gross JA, Johnston J, Mudri S, Enselman R, Dillon SR, Madden K, Xu W, Parrish-Novak J, Foster D, Lofton-Day C, Moore M, Littau A, Grossman A, Haugen H, Foley K, Blumberg H, Harrison K, Kindsvogel W, Clegg CH. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature. 2000;404:995–999. doi: 10.1038/35010115. [DOI] [PubMed] [Google Scholar]
- 16.Mackay F, Woodcock SA, Lawton P, Ambrose C, Baetscher M, Schneider P, Tschopp J, Browning JL. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999;190:1697–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rahman ZS, Rao SP, Kalled SL, Manser T. Normal induction but attenuated progression of germinal center responses in BAFF and BAFF-R signaling-deficient mice. J Exp Med. 2003;198:1157–1169. doi: 10.1084/jem.20030495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vora KA, Wang LC, Rao SP, Liu ZY, Majeau GR, Cutler AH, Hochman PS, Scott ML, Kalled SL. Cutting edge: germinal centers formed in the absence of B cell-activating factor belonging to the TNF family exhibit impaired maturation and function. J Immunol. 2003;171:547–551. doi: 10.4049/jimmunol.171.2.547. [DOI] [PubMed] [Google Scholar]
- 19.Yang X, Lee J, Mahony EM, Kwong PD, Wyatt R, Sodroski J. Highly stable trimers formed by human immunodeficiency virus type 1 envelope glycoproteins fused with the trimeric motif of T4 bacteriophage fibritin. J Virol. 2002;76:4634–4642. doi: 10.1128/JVI.76.9.4634-4642.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forsell MN, Dey B, Morner A, Svehla K, O’Dell S, Hogerkorp CM, Voss G, Thorstensson R, Shaw GM, Mascola JR, Karlsson Hedestam GB, Wyatt RT. B cell recognition of the conserved HIV-1 co-receptor binding site is altered by endogenous primate CD4. PLoS Pathog. 2008;4:e1000171. doi: 10.1371/journal.ppat.1000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dosenovic P, Chakrabarti B, Soldemo M, Douagi I, Forsell MN, Li Y, Phogat A, Paulie S, Hoxie J, Wyatt RT, Karlsson Hedestam GB. Selective expansion of HIV-1 envelope glycoprotein-specific B cell subsets recognizing distinct structural elements following immunization. J Immunol. 2009;183:3373–3382. doi: 10.4049/jimmunol.0900407. [DOI] [PubMed] [Google Scholar]
- 22.Li M, Gao F, Mascola JR, Stamatatos L, Polonis VR, Koutsoukos M, Voss G, Goepfert P, Gilbert P, Greene KM, Bilska M, Kothe DL, Salazar-Gonzalez JF, Wei X, Decker JM, Hahn BH, Montefiori DC. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol. 2005;79:10108–10125. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shu Y, Winfrey S, Yang ZY, Xu L, Rao SS, Srivastava I, Barnett SW, Nabel GJ, Mascola JR. Efficient protein boosting after plasmid DNA or recombinant adenovirus immunization with HIV-1 vaccine constructs. Vaccine. 2007;25:1398–1408. doi: 10.1016/j.vaccine.2006.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morner A, Douagi I, Forsell MN, Sundling C, Dosenovic P, O’Dell S, Dey B, Kwong PD, Voss G, Thorstensson R, Mascola JR, Wyatt RT, Karlsson Hedestam GB. Human immunodeficiency virus type 1 env trimer immunization of macaques and impact of priming with viral vector or stabilized core protein. J Virol. 2009;83:540–551. doi: 10.1128/JVI.01102-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiang SH, Kwong PD, Gupta R, Rizzuto CD, Casper DJ, Wyatt R, Wang L, Hendrickson WA, Doyle ML, Sodroski J. Mutagenic stabilization and/or disruption of a CD4-bound state reveals distinct conformations of the human immunodeficiency virus type 1 gp120 envelope glycoprotein. J Virol. 2002;76:9888–9899. doi: 10.1128/JVI.76.19.9888-9899.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng Y, McKee K, Tran K, O’Dell S, Schmidt SD, Phogat A, Forsell MN, Karlsson Hedestam GB, Mascola JR, Wyatt RT. Biochemically defined HIV-1 Env variant immunogens display differential binding and neutralizing specificities to the CD4 binding site. J Biol Chem. 2011 doi: 10.1074/jbc.M111.317776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore PA, Belvedere O, Orr A, Pieri K, LaFleur DW, Feng P, Soppet D, Charters M, Gentz R, Parmelee D, Li Y, Galperina O, Giri J, Roschke V, Nardelli B, Carrell J, Sosnovtseva S, Greenfield W, Ruben SM, Olsen HS, Fikes J, Hilbert DM. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science. 1999;285:260–263. doi: 10.1126/science.285.5425.260. [DOI] [PubMed] [Google Scholar]
- 28.Ota M, Duong BH, Torkamani A, Doyle CM, Gavin AL, Ota T, Nemazee D. Regulation of the B cell receptor repertoire and self-reactivity by BAFF. J Immunol. 2010;185:4128–4136. doi: 10.4049/jimmunol.1002176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khare SD, Sarosi I, Xia XZ, McCabe S, Miner K, Solovyev I, Hawkins N, Kelley M, Chang D, Van G, Ross L, Delaney J, Wang L, Lacey D, Boyle WJ, Hsu H. Severe B cell hyperplasia and autoimmune disease in TALL-1 transgenic mice. Proc Natl Acad Sci U S A. 2000;97:3370–3375. doi: 10.1073/pnas.050580697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gor DO, Ding X, Li Q, Sultana D, Mambula SS, Bram RJ, Greenspan NS. Enhanced immunogenicity of pneumococcal surface adhesin A (PsaA) in mice via fusion to recombinant human B lymphocyte stimulator (BLyS) Biol Direct. 2011;6:9. doi: 10.1186/1745-6150-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melchers M, Bontjer I, Tong T, Chung NP, Klasse PJ, Eggink D, Montefiori DC, Gentile M, Cerutti A, Olson WC, Berkhout B, Binley JM, Moore JP, Sanders RW. Targeting HIV-1 envelope glycoprotein trimers to B cells using APRIL improves antibody responses. J Virol. 2011 doi: 10.1128/JVI.06259-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kanagavelu SK, Snarsky V, Termini JM, Gupta S, Barzee S, Wright JA, Khan WN, Kornbluth RS, Stone GW. Soluble multi-trimeric TNF superfamily ligand adjuvants enhance immune responses to a HIV-1 Gag DNA vaccine. Vaccine. 2012;30:691–702. doi: 10.1016/j.vaccine.2011.11.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verkoczy L, Chen Y, Bouton-Verville H, Zhang J, Diaz M, Hutchinson J, Ouyang YB, Alam SM, Holl TM, Hwang KK, Kelsoe G, Haynes BF. Rescue of HIV-1 Broad Neutralizing Antibody-Expressing B Cells in 2F5 VH × VL Knockin Mice Reveals Multiple Tolerance Controls. J Immunol. 2011;187:3785–3797. doi: 10.4049/jimmunol.1101633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scherer EM, Zwick MB, Teyton L, Burton DR. Difficulties in eliciting broadly neutralizing anti-HIV antibodies are not explained by cardiolipin autoreactivity. AIDS. 2007;21:2131–2139. doi: 10.1097/QAD.0b013e3282a4a632. [DOI] [PubMed] [Google Scholar]
- 35.Scherer EM, Leaman DP, Zwick MB, McMichael AJ, Burton DR. Aromatic residues at the edge of the antibody combining site facilitate viral glycoprotein recognition through membrane interactions. Proc Natl Acad Sci U S A. 2010;107:1529–1534. doi: 10.1073/pnas.0909680107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liao HX, Chen X, Munshaw S, Zhang R, Marshall DJ, Vandergrift N, Whitesides JF, Lu X, Yu JS, Hwang KK, Gao F, Markowitz M, Heath SL, Bar KJ, Goepfert PA, Montefiori DC, Shaw GC, Alam SM, Margolis DM, Denny TN, Boyd SD, Marshal E, Egholm M, Simen BB, Hanczaruk B, Fire AZ, Voss G, Kelsoe G, Tomaras GD, Moody MA, Kepler TB, Haynes BF. Initial antibodies binding to HIV-1 gp41 in acutely infected subjects are polyreactive and highly mutated. J Exp Med. 2011;208:2237–2249. doi: 10.1084/jem.20110363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morris L, Chen X, Alam M, Tomaras G, Zhang R, Marshall DJ, Chen B, Parks R, Foulger A, Jaeger F, Donathan M, Bilska M, Gray ES, Abdool Karim SS, Kepler TB, Whitesides J, Montefiori D, Moody MA, Liao HX, Haynes BF. Isolation of a human anti-HIV gp41 membrane proximal region neutralizing antibody by antigen-specific single B cell sorting. PLoS One. 2011;6:e23532. doi: 10.1371/journal.pone.0023532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsu BL, Harless SM, Lindsley RC, Hilbert DM, Cancro MP. Cutting edge: BLyS enables survival of transitional and mature B cells through distinct mediators. J Immunol. 2002;168:5993–5996. doi: 10.4049/jimmunol.168.12.5993. [DOI] [PubMed] [Google Scholar]
- 39.Batten M, Groom J, Cachero TG, Qian F, Schneider P, Tschopp J, Browning JL, Mackay F. BAFF mediates survival of peripheral immature B lymphocytes. J Exp Med. 2000;192:1453–1466. doi: 10.1084/jem.192.10.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kalled SL. Impact of the BAFF/BR3 axis on B cell survival, germinal center maintenance and antibody production. Semin Immunol. 2006;18:290–296. doi: 10.1016/j.smim.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 41.Chen X, Martin F, Forbush KA, Perlmutter RM, Kearney JF. Evidence for selection of a population of multi-reactive B cells into the splenic marginal zone. Int Immunol. 1997;9:27–41. doi: 10.1093/intimm/9.1.27. [DOI] [PubMed] [Google Scholar]
- 42.Carey JB, Moffatt-Blue CS, Watson LC, Gavin AL, Feeney AJ. Repertoire-based selection into the marginal zone compartment during B cell development. J Exp Med. 2008;205:2043–2052. doi: 10.1084/jem.20080559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lesley R, Xu Y, Kalled SL, Hess DM, Schwab SR, Shu HB, Cyster JG. Reduced competitiveness of autoantigen-engaged B cells due to increased dependence on BAFF. Immunity. 2004;20:441–453. doi: 10.1016/s1074-7613(04)00079-2. [DOI] [PubMed] [Google Scholar]
- 44.Thorn M, Lewis RH, Mumbey-Wafula A, Kantrowitz S, Spatz LA. BAFF overexpression promotes anti-dsDNA B-cell maturation and antibody secretion. Cell Immunol. 2010;261:9–22. doi: 10.1016/j.cellimm.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Malaspina A, Moir S, Ho J, Wang W, Howell ML, O’Shea MA, Roby GA, Rehm CA, Mican JM, Chun TW, Fauci AS. Appearance of immature/transitional B cells in HIV-infected individuals with advanced disease: correlation with increased IL-7. Proc Natl Acad Sci U S A. 2006;103:2262–2267. doi: 10.1073/pnas.0511094103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moir S, Malaspina A, Pickeral OK, Donoghue ET, Vasquez J, Miller NJ, Krishnan SR, Planta MA, Turney JF, Justement JS, Kottilil S, Dybul M, Mican JM, Kovacs C, Chun TW, Birse CE, Fauci AS. Decreased survival of B cells of HIV-viremic patients mediated by altered expression of receptors of the TNF superfamily. J Exp Med. 2004;200:587–599. [PubMed] [Google Scholar]
- 47.Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien JP, Wang SK, Ramos A, Chan-Hui PY, Moyle M, Mitcham JL, Hammond PW, Olsen OA, Phung P, Fling S, Wong CH, Phogat S, Wrin T, Simek MD, Koff WC, Wilson IA, Burton DR, Poignard P PG Principal Investigators. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scheid JF, Mouquet H, Ueberheide B, Diskin R, Klein F, Olivera TY, Pietzsch J, Fenyo D, Abadir A, Velinzon K, Hurley A, Myung S, Boulad F, Poignard P, Burton D, Pereyra F, Ho DD, Walker BD, Seaman MS, Bjorkman PJ, Chait BT, Nussenzweig MC. Sequence and Structural Convergence of Broad and Potent HIV Antibodies That Mimic CD4 Binding. Science. 2011 doi: 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu X, Yang ZY, Li Y, Hogerkorp CM, Schief WR, Seaman MS, Zhou T, Schmidt SD, Wu L, Xu L, Longo NS, McKee K, O’Dell S, Louder MK, Wycuff DL, Feng Y, Nason M, Doria-Rose N, Connors M, Kwong PD, Roederer M, Wyatt RT, Nabel GJ, Mascola JR. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010;329:856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.