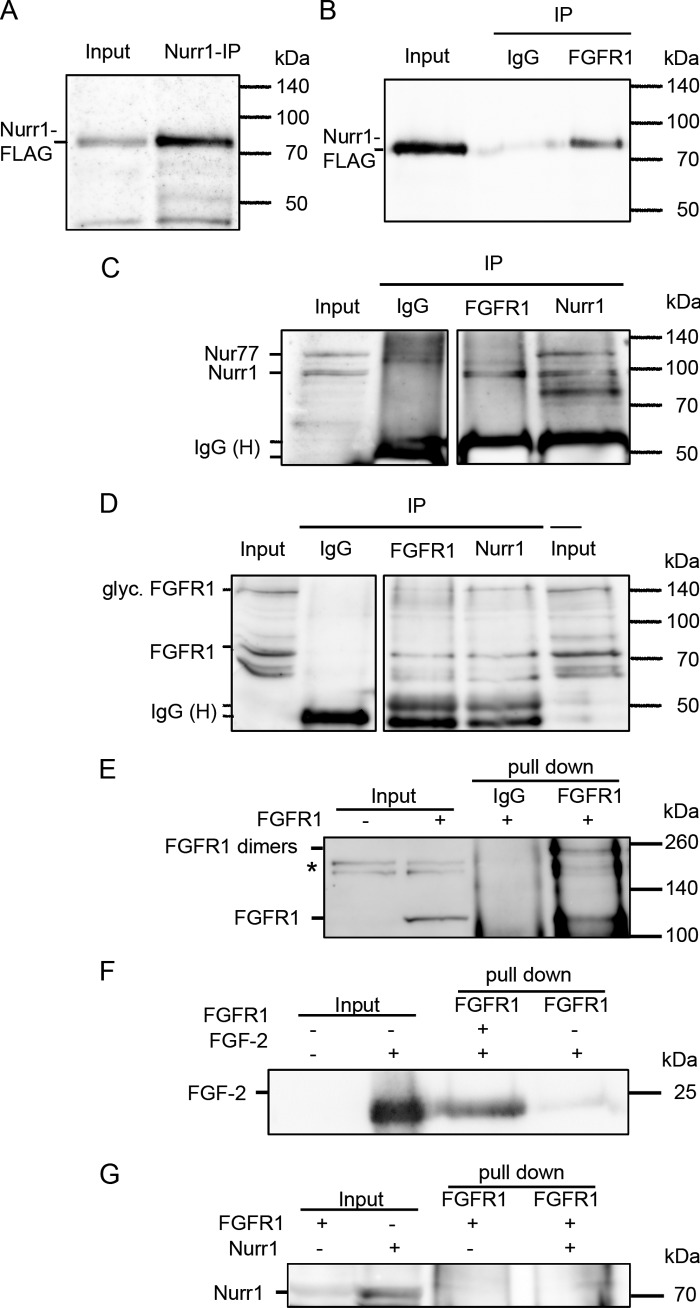
FIGURE 4.
Nuclear FGFR1 and Nurr1 interaction after overexpression in human neuroblastoma cells. The human neuroblastoma cells were transfected with plasmids encoding for full-length FGFR1 protein, as well as Nurr1-protein fused to a 3×FLAG tag. 24 h after transfection, the cells were supplemented with 1 μm retinoic acid for a further 24 h. A–D, the nuclear extracts were immunoprecipitated with polyclonal anti-Nurr1, anti-FGFR1, and rabbit IgGs as negative control. Input represents 25 μg (A and C) and 100 μg (B and C), respectively, protein of the not precipitated nuclear extract. The additional band in C (compare Fig. 3K) may represent the closely related Nur77, which shows only a faint expression in VM (47). Precipitation with polyclonal Nurr1 and FGFR1 antibodies functioned properly as shown by detection of precipitated Nurr1 with the anti-FLAG antibody (A) and of precipitated FGFR1 with monoclonal anti-FGFR1 (mAb6) antibody (D), respectively. The FGFR1-IP resulted in co-precipitation of Nurr1 as recognized by anti-FLAG-tag antibody (B), as well as with anti-Nurr1 antibody (C, represents one blot). Correspondingly, the ∼85-kDa form of FGFR1 was able to co-precipitate with Nurr1, as detected by monoclonal anti-FGFR1 (mAb6) antibody (D, represents one blot). The negative controls, precipitated with rabbit IgGs, were missing the specific bands, confirming a specificity of the Nurr1 and FGFR1 immunoprecipitations. E, in vitro coupled transcription/translation of FGFR1 resulted in positive product at ∼120 kDa (input), which was missing in the control translation reaction without DNA template. The subsequent pull-down of FGFR1 with anti-FGFR1 antibody resulted in positive precipitates at ∼120 and ∼250 kDa, which would correspond to FGFR1 dimers. *, unspecific cross-reaction only observed in reticulocyte extracts. F, the positive interaction of FGF-2 was confirmed by subsequent pull-down along with FGFR1. G, the interaction of Nurr1 and FGFR1 seems to be indirect, because the subsequent pull-downs of Nurr1 and FGFR1 were negative.