FIGURE 1.
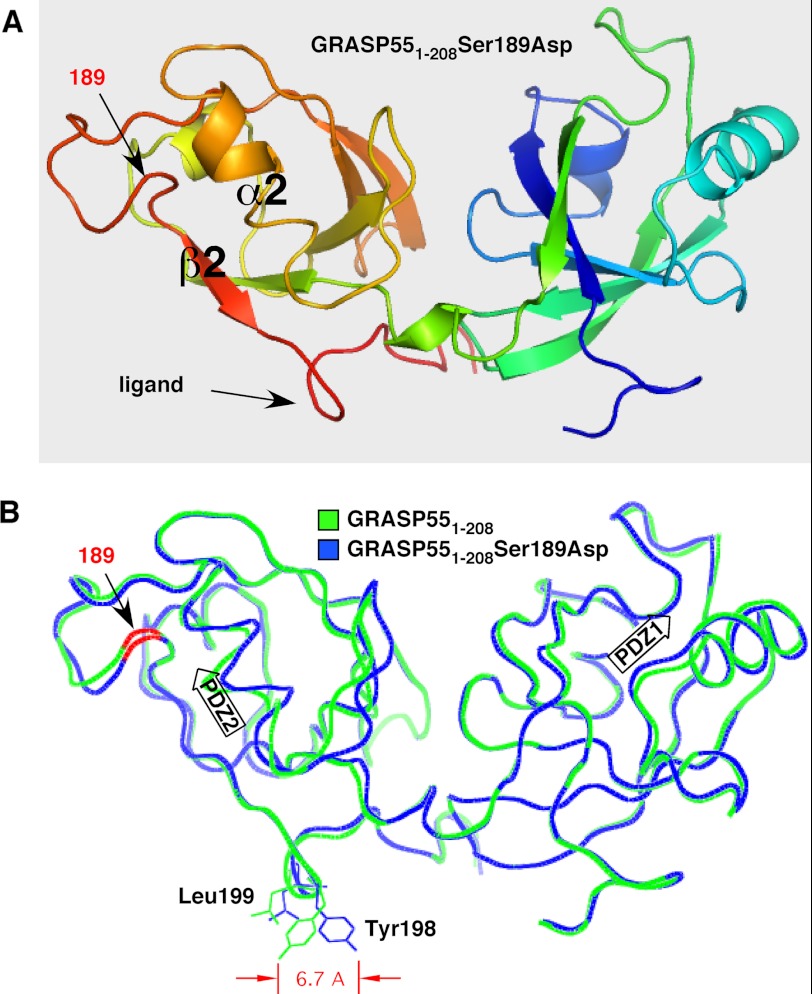
Structure of GRASP domain phosphomimic S189D and comparison with non-phosphorylated GRASP domain. A, schematic structure of GRASP55(1–208) containing the S189D substitution showing the two PDZ domains PDZ1 (right) and PDZ2 (left), with key features of the latter highlighted. These are the α2-helix and β2-strand that define the pocket, the surface feature corresponding to the internal PDZ ligand, and the position of the aspartic acid that was substituted for serine. Coloring reflects N-terminal (blue) to C-terminal (red) progression. B, comparison of GRASP55(1–208) S189D (blue) and GRASP55(1–208) (green) shows high overall similarity with one another and a 6.7-Å displacement of Tyr-198. Together with Leu-199 (also shifted), Tyr-198 composes the surface protuberance of the internal PDZ ligand. The positions of the PDZ1 and PDZ2 binding pockets are indicated by arrows, and the location of residue 189 on the backbone is colored red.