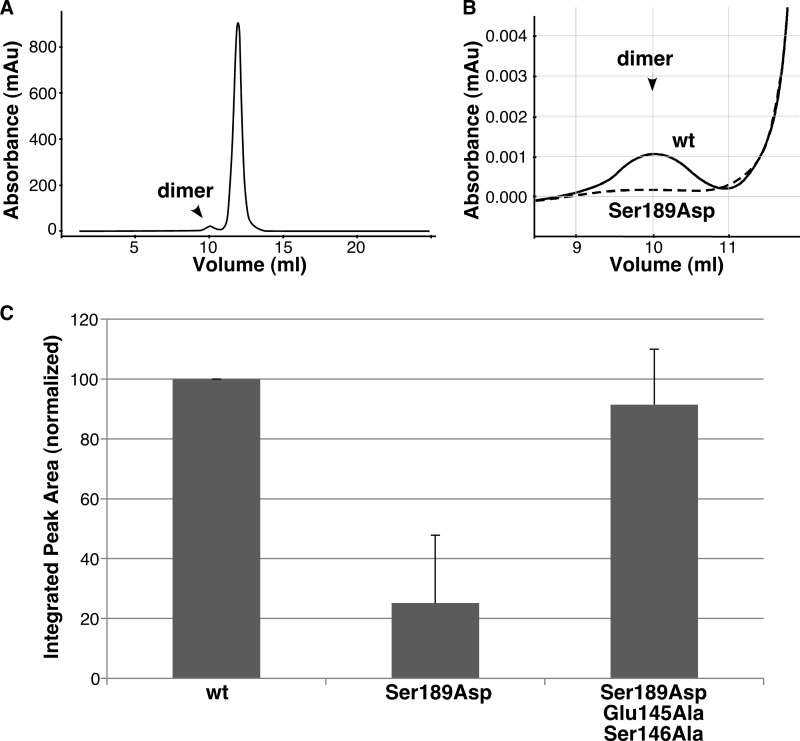
FIGURE 4.
Phosphomimic mutation disrupts dimerization by means of Glu-145 and Ser-146. A, Superdex G-75 elution profile of GRASP55(1–208), with the position of the minor dimer fraction indicated. mAu, milli-absorbance units. B, enlarged view of the dimer fraction for GRASP55(1–208) compared with that for GRASP55(1–208) S189D. C, quantified recovery of GRASP55(1–208), GRASP55(1–208) S189D, and GRASP55(1–208) S189D/E145A/S146A in the dimer fraction (mean ± S.D., n = 3). Note that the phosphomimic mutation blocked binding, but the block required the residues that sit between the phosphorylation site and the ligand and reverse position in the phosphomimic.