FIGURE 5.
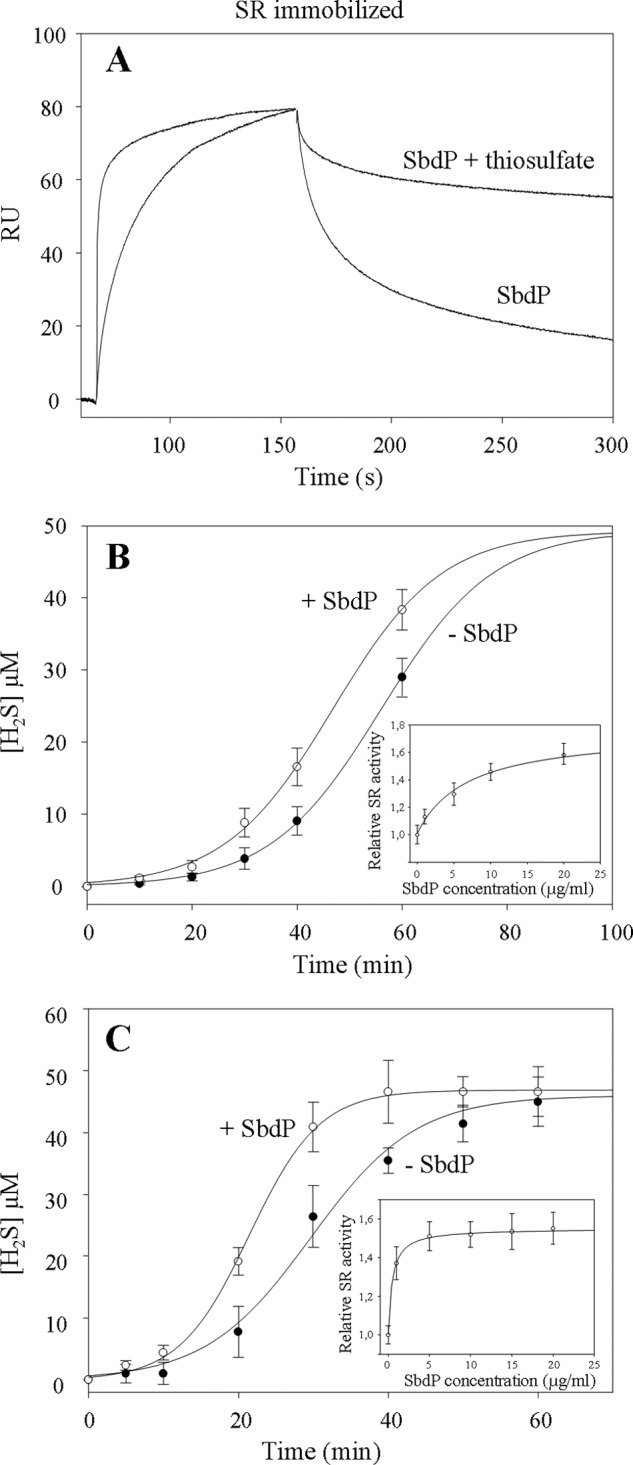
Investigation of SbdP-SR complex partnership. A, SPR experiment; SR complex was immobilized at 4710 RU on a CM5 sensor chip, and SbdP (0.6 μg) incubated or not with 10 mm thiosulfate (S2O32−) was injected. Thiosulfate alone was also injected as a control, without modification of the signal. B and C, sulfur reductase activity measurements of SR complex (50 μg·ml−1, final concentration) (B) or solubilized NaBr-washed membranes (50 μg·ml−1, final concentration) (C) performed with the insoluble S0 in presence (white circles) or absence (black circles) of exogenous SbdP (5 μg·ml−1, final concentration) as well as in presence of increasing concentrations of SbdP (1, 5, 10, 15, and 20 μg·ml−1, final concentration) (inset, B and C) after 40 min of incubation. Ratio between the different proteins, however, cannot be calculated because the oligomerization state of the active form of both proteins is yet unknown. A clear concentration dependence illustrates that we are most likely not in an excess of one over the other. Results presented correspond to an average of three independent experiments.
