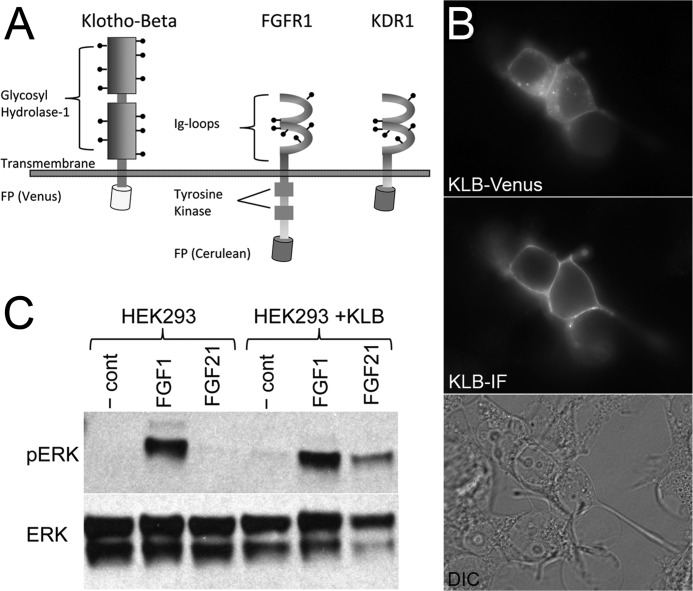
FIGURE 1.
Expression of fluorescent protein-tagged KLB and FGFR1 constructs. A, a schematic of fluorescent protein (FP)-tagged human KLB and FGFR1 fluorescent protein-tagged constructs. Human KLB (UniProt, Q86Z14) has a large extracellular domain (996 amino acids) with 11 putative N-glycosylation sites (indicated by stick and ball markings) and a short intracellular domain (27 amino acids). Human FGFR1c (UniProt, P11362-3) has a 265-amino acid extracellular domain with two IgG-like loops, six putative N-glycosylation sites, and a significantly longer intracellular domain (424 amino acids). The kinase-deficient receptor construct (KDR1) has a truncated intercellular domain with only 20 amino acids between the transmembrane domain and start of the fluorescent protein (depicted as a barrel). B, HeLa cells expressing KLB-Venus were fixed and immunofluorescently labeled for the extracellular domain of KLB. The top panel demonstrates Venus-associated fluorescence throughout the cell and at the plasma membrane. The middle panel demonstrates KLB-associated immunofluorescence in nonpermeabilized cells indicative of receptor detected at the plasma membrane. Cells in the entire field of view are shown in the differential interference contrast (DIC) image at the bottom. C, representative FGF1- and FGF21-induced phospho-ERK1/2 (pERK) activation detected by Western immunoblot. HEK293T cells endogenously express FGFR1 but do not express KLB. These cells respond to FGF1 (10 ng/ml) but are nonresponsive to FGF21 (100 ng/ml). In contrast, HEK293T overexpressing the KLB-Venus construct are responsive to both FGF1 (10 ng/ml) and FGF21 (100 ng/ml).