FIGURE 5.
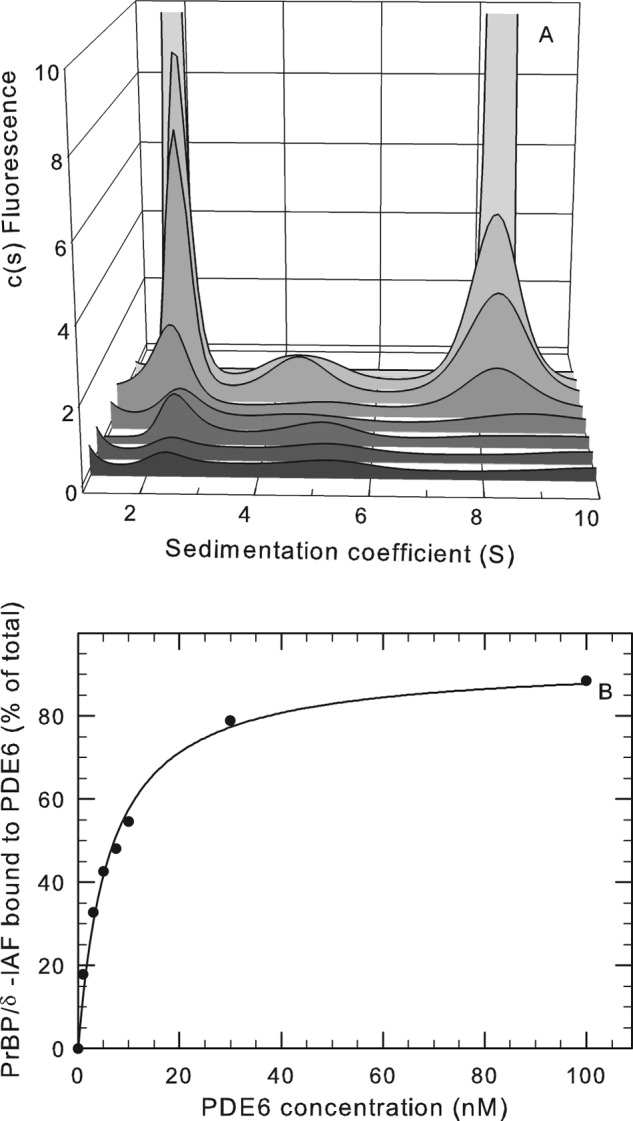
Binding of PDE6-interacting protein PrBP/δ with PDE6 holoenzyme. A, purified PrBP/δ-IAF (1 μm) was incubated overnight at 4 °C with 420 nm PDE6 holoenzyme, and then unbound PrBP/δ-IAF was separated from PDE6 by gel filtration chromatography. The recovered protein complex was then diluted to the following PDE6 concentrations (listed from front to back): 1, 3, 5, 7.5, 10, 30, and 100 nm. Sedimentation velocity analysis of the dilution series was then performed, along with a control sample (not shown) consisting of purified PrBP/δ-IAF, which sediments at 2.0 S. At 100 nm, the amplitude of the fluorescent signal (2 S peak = 29.4 units and 8 S peak = 113 units) is off scale so that the results at lower concentrations can be seen. B, the percentage of the total PrBP/δ fluorescence associated with PDE6 was determined for each PDE6 concentration by integrating the areas under the observed peaks and calculating the percentage of the total fluorescence migrating at 8.1 S. The curve represents the fit of the data to a two-parameter hyperbolic function (K½ = 6.1 nm).
