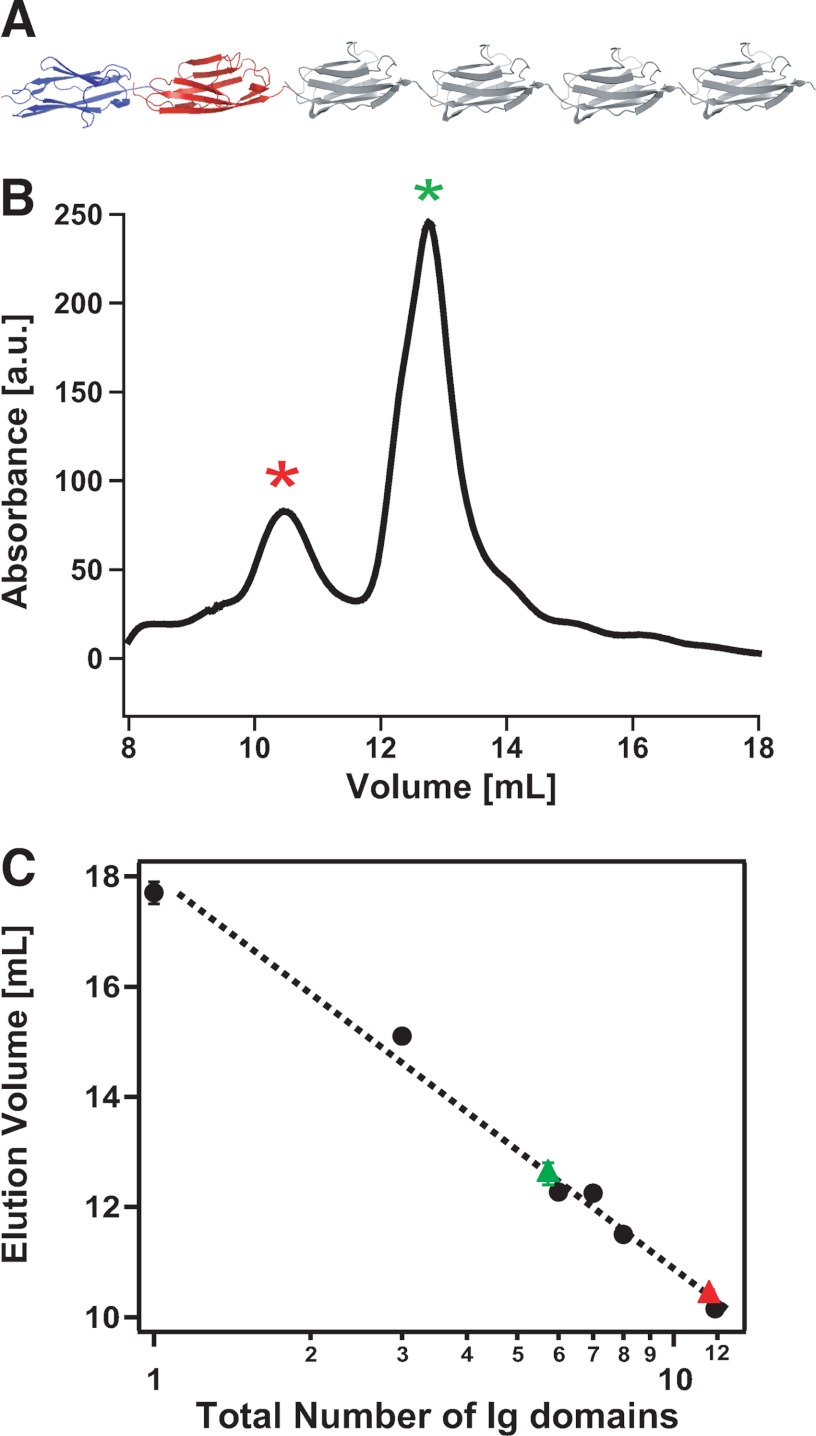
FIGURE 2.
The (I27)4Z1Z2 protein spontaneously forms dimers in solution. A, diagram of the engineered (I27)4Z1Z2 polyprotein. B, size-exclusion chromatogram of the (I27)4Z1Z2 protein, exhibiting two well defined peaks, the first one occurring at ∼10.4 ml (red asterisk) and the second one occurring at a higher elution volume of ∼12.6 ml (green asterisk). a. u., arbitrary units. C, representation of the elution volume as a function of the size of the protein (in logarithmic scale). The Z1 and Z2 Ig domains of titin have approximately the same size (99 and 100 amino acids, respectively) as the I27 Ig module of titin (89 amino acids). With calibration purposes, we used I27 polyproteins prepared using the same expression system but composed of a varying number of monomers: (I27)n. Specifically, for calibration of the column, we used I27 polyproteins composed of n = 1, 3, 6, 7, 8, or 12 I27 domains. Interpolation of the elution volumes corresponding to the two peaks indicate that the first peak corresponds to the size of a 12-mer (∼11 kDa/domain), thus consistent with a dimeric 2((I27)4Z1Z2) protein, and the second peak corresponds to a 6-mer, suggestive of a monomeric (I27)4Z1Z2 protein.