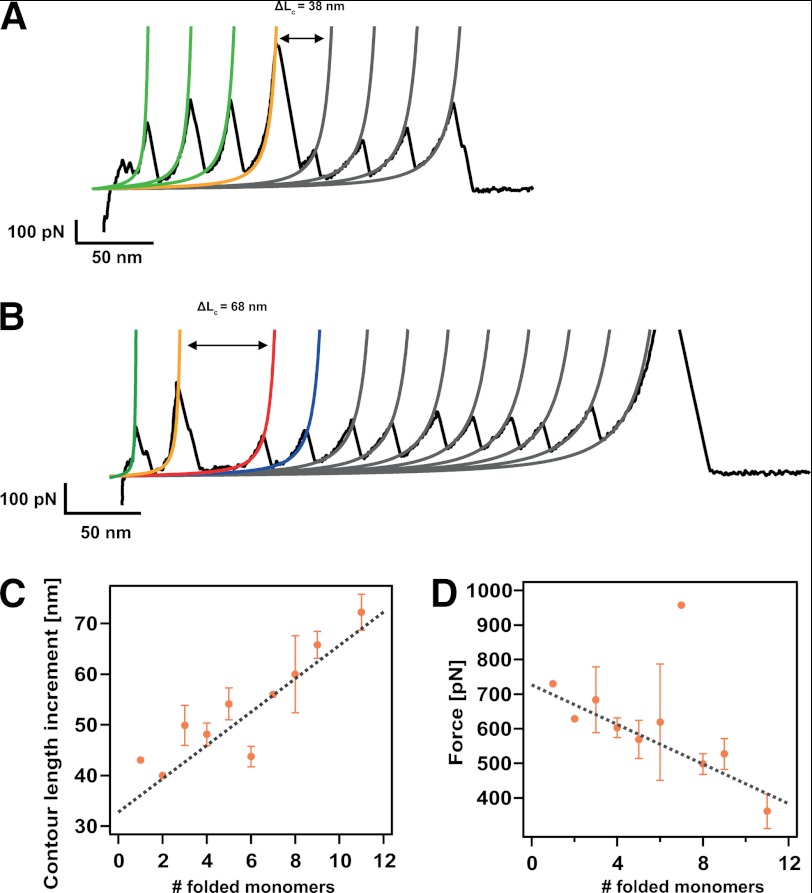
FIGURE 5.
The molecular mechanism of dimer disruption involves unfolding of one protein domain and extension of the domains that still remained folded. A, typical unfolding trajectory where three domains are still folded once the forced-induce rupture occurs, eliciting an increase in length of 38 nm. B, unfolding trajectory where the dimer-to-monomer transition occurs when nine monomers remained folded. In this case, such disruption entails a longer increment of released length of ΔL = 68 nm. C, dependence of the increment in contour length, ΔL, associated with the dimer-to-monomer transition as a function of the folded monomers, showing a linear dependence. Linear fit to the data (gray discontinuous line) yielded a slope of 3.3 nm (close to the size of a folded monomer) and an intercept of 32.8 nm (corresponding to the unfolding of one monomer in the chain). D, dependence of the force at which the dimer-to-monomer transition occurs with the number of folded modules remaining in the chain. The negative linear correlation indicates that on average, the presence of each extra monomer in the chain decreases the mechanical stability of the complex by ∼29 pN (linear fit, gray line). Error bars stand for S.E. in each case. The individual data points devoid of error bars correspond to measurements stemming from an individual unfolding trajectory.