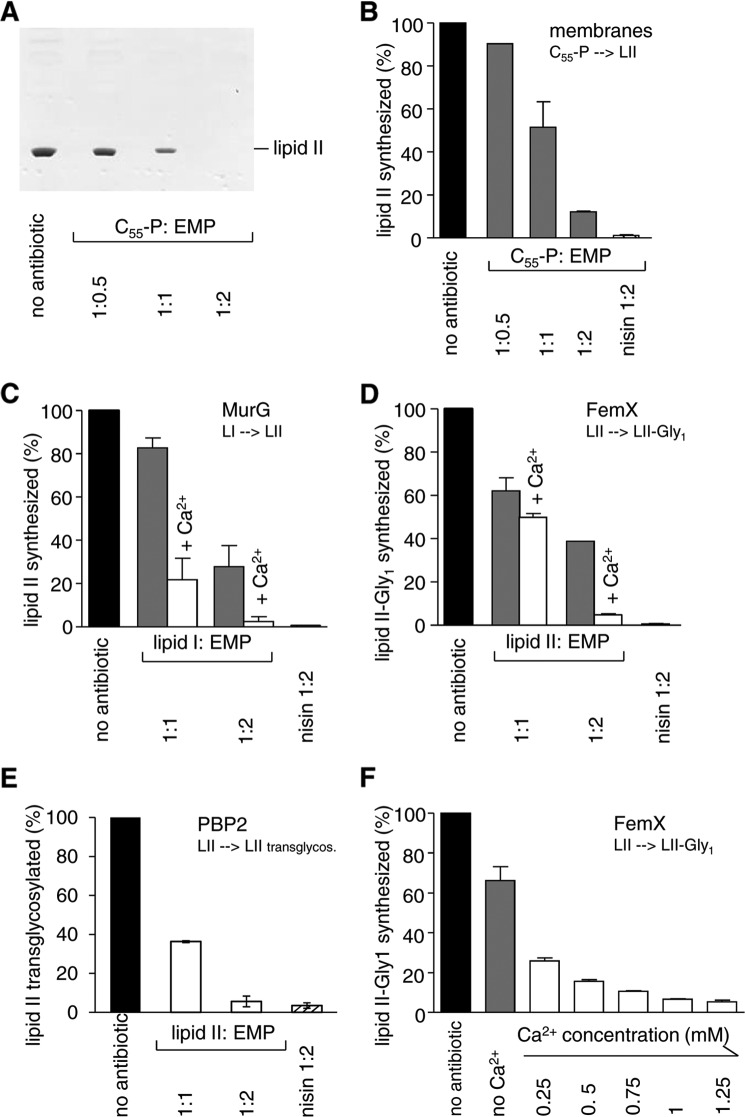
FIGURE 4.
Effect of empedopeptin on membrane-associated stages of peptidoglycan synthesis in vitro. A and B, in vitro lipid II synthesis catalyzed by M. luteus membrane preparations. A, thin layer chromatography of n-butanol/pyridine acetate extracts and detection by phosphomolybdic acid staining. B, quantification of synthesized lipid II in n-butanol/pyridine acetate extracts of the membrane preparation via incorporated [14C]UDP-GlcNAc and phosphorimaging. C, D, E, and F, reactions catalyzed by purified enzymes from purified substrates. C, lipid II synthesis from lipid I by MurG quantified via incorporated [14C]UDP-GlcNAc. D and F, lipid II-Gly1 synthesis from lipid II by FemX quantified via incorporated [14C]glycine. E, the transglycosylation reaction catalyzed by PBP2 was quantified by the reduction of free [14C]lipid II. The inhibitory activity of empedopeptin in the absence (gray bars) or presence (white bars) of 1.25 mm Ca2+ (C, D, and E) or in the presence of a calcium gradient (F) is shown. The FemX reaction was chosen as an exemplary system to probe the effect of a calcium concentration range. For all reactions, the amount of products synthesized by control reactions in the absence of antibiotic was set to 100%. Empedopeptin was added at molar ratios of 0.5:1, 1:1, and 2:1 with respect to the lipid substrates as indicated. In F, the molar ratio of lipid II:empedopeptin was 1:2. The lantibiotic nisin in a 2-fold molar excess served as a control. Mean values from three independent experiments are shown. Error bars indicate the standard deviation. EMP, empedopeptin; LI, lipid I; LII, lipid II.