FIGURE 2.
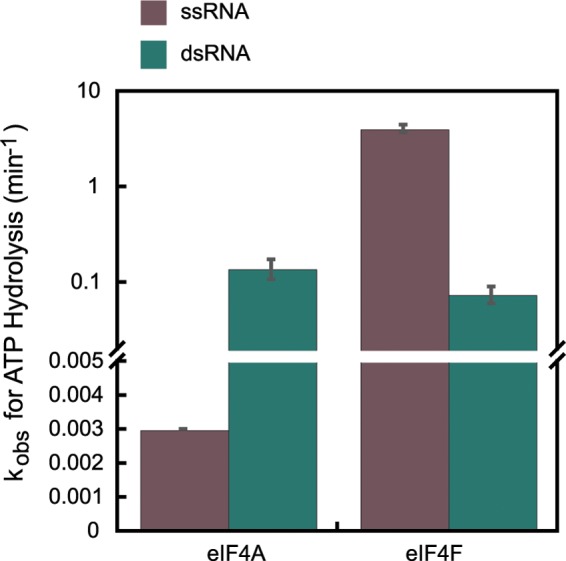
Steady-state ATPase activities of eIF4A and eIF4F. ATPase activities of WT eIF4A and eIF4F were measured as described under “Experimental Procedures.” Briefly, ATP (5 mm) was added to the reaction mixture containing eIF4A or eIF4F (200 nm) with saturating single-stranded or double-stranded nucleic acids substrates (5 μm). The reaction was allowed to proceed for 0–180 min at 26 °C in Recon buffer. The reaction was quenched with formic acid, and the products were resolved by polyethyleneimine-cellulose TLC and quantified using ImageQuant. The molar ATP hydrolyzed per molar enzyme was plotted as a function of time. The data were fit with an equation for a straight line. The slope of this line gave the initial rate of ATP hydrolysis. The ATPase rates reported here have been corrected for the low level of background ATPase observed in the absence of any nucleic acid (see “Experimental Procedures”).
