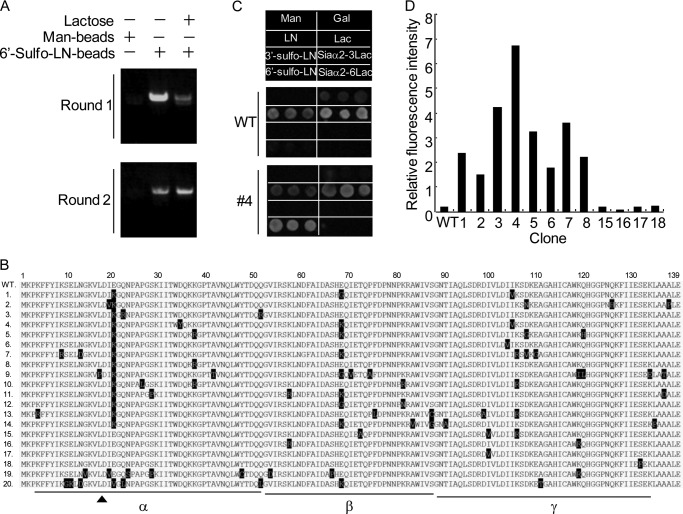
FIGURE 1.
Selection of 6S-Gal binding lectins. A, selection of 6′-Sulfo-LN binding lectins by pretreatment of the translation mixture with excessive lactose. The EW29Ch library generated by error-prone PCR was selected with 6′-Sulfo-LN-coated beads with or without pretreatment of the translation mixture with lactose. The precipitated mRNAs under individual conditions were isolated and reverse-transcribed to cDNA by one-step RT-PCR. A part of cDNA was used for analysis by agarose electrophoresis. The rest of the cDNAs selected in the presence of lactose were subsequently used for the second round of ribosome display. In all experiments, mannose-polyacrylamide was used as a negative control. B, alignment of the amino acid sequences of 20 selected mutants along with the WT. The mutated amino acids are highlighted on black background. Three subdomains of EW29Ch, α, β, and γ, are underlined. The key residue Asp-18 in the binding with Gal is indicated by arrows. C and D, analysis of the sugar-binding activity of selected mutants by glycoconjugate microarray. Twelve mutants with an N-terminal FLAG tag (1–8 and 15–18) were expressed in E. coli and lysed. The resulting lysates were Cy3-labeled and then applied to a glass slide where eight kinds of glycans were immobilized. The binding of mutants to individual glycans was monitored by an evanescent-type scanner. C, the binding profile of wild-type EW29Ch and a representative mutant (#4). The relative signal of 6′-Sulfo-LN to LN of 12 expressing mutants is indicated in D.