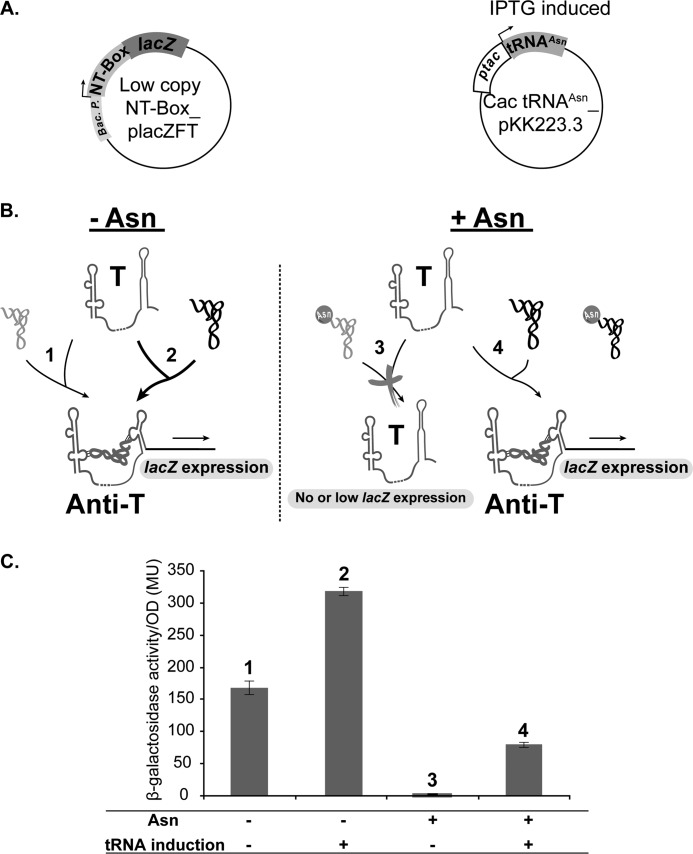
FIGURE 8.
Design and analysis of in vivo NT-box-mediated antitermination assay in Gram-negative E. coli bacterium. A, schematic representation of the in vivo antitermination system in E. coli. The E. coli ER strain was co-transformed with the NT-box_placZFT and Cac tRNAAsn_pKK223-3 recombinant plasmids. “Bac. P.” corresponds to the endogenous NT-box promoter. “ptac” corresponds to the IPTG-inducible promoter controlling Cac tRNAAsn gene expression. B, schematic representation of the NT-box-controlled lacZ expression in E. coli under the four conditions tested (see C) using the two RNAs: Cac NT-Box and tRNAAsn (colored in black). The endogenous E. coli tRNAAsn (colored in light gray) has a “GUU” anticodon sequence. The tRNAAsn colored in dark gray and bound to the NT-box corresponds to the E. coli tRNAAsn and Cac uncharged tRNAAsn, which both can affect antitermination. T, NT-box terminator conformation; Anti-T, NT-box antiterminator conformation. C, in vivo NT-box-mediated antitermination assay. Bars for each graph represent β-galactosidase activity in Miller units (MU) relative to cell density (53). Error bars = Mean of β-galactosidase activity/cell density of three independent activity measurement replicates ± S.D. The effect of Asn presence and tRNA induction were compared by taking 5-ml aliquots from each culture and measuring the β-galactosidase activity after 4 h of culture. The β-galactosidase activity value was calculated using the following equation: 1000 × ((A420 − (1.75 × A560))/(T × V × A595)) where “A420” is the absorbance of the o-nitrophenol product (see “Experimental Procedures”), “A560” is the absorbance of the cell debris pellet, “A595” is the absorbance of bacterial suspension, “T” is the reaction time in minutes, and “V” is the volume in milliliters of the treated cells used to measure β-galactosidase activity.