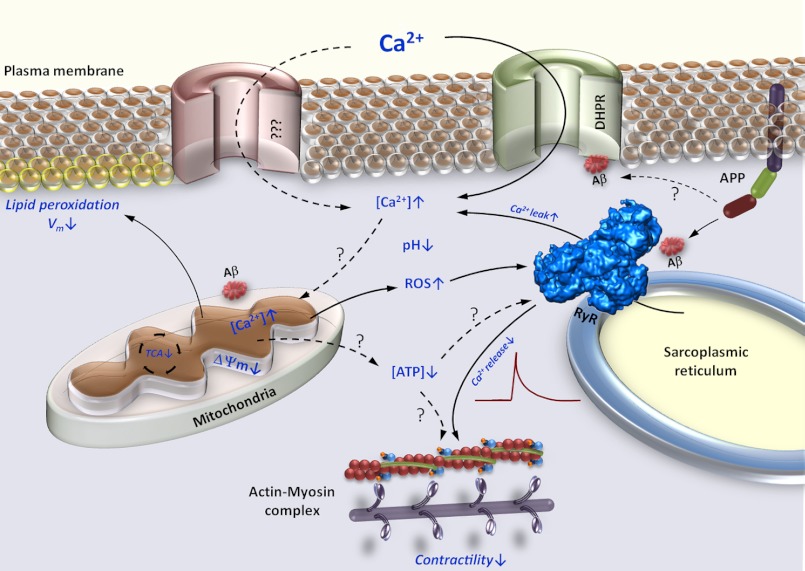
FIGURE 8.
Proposed model of β-amyloid mediated muscle dysfunction in IBM. Increased expression of APP and accumulation of its proteolytic Aβ fragments affects numerous intracellular regulatory complexes. Modulation of the intracellular Ca2+ release channels (RyR), dihydropyridine receptors (DHPR) and possibly other surface membrane Ca2+ channels leads to Ca2+ efflux and influx, respectively, which contribute to augmentation of the resting cytoplasmic Ca2+ concentration ([Ca2+]i). Aβ accumulation also leads to decreased Ca2+ release by RyRs. Augmented [Ca2+]i together with modification of mitochondrial function by Aβ lead to enhanced Ca2+ flux into the mitochondrial matrix and subsequent mitochondrial Ca2+ overload. Mitochondrial Ca2+ overload promotes enhanced generation of ROS, mitochondrial permeability transition pore complex opening, and disruption of ΔΨm. Diminished ΔΨm results in inefficient TCA cycle and reduction in mitochondrial ATP production. Increased ROS production in combination with acidic cytoplasm promotes lipid peroxidation, further increase in [Ca2+]i, and reduction in Ca2+ release from the SR through modulation of the RyRs. All of these events combine to lead to diminished muscle contractility. Solid arrows represent observed, whereas dashed arrows represent unconfirmed effects of Aβ.