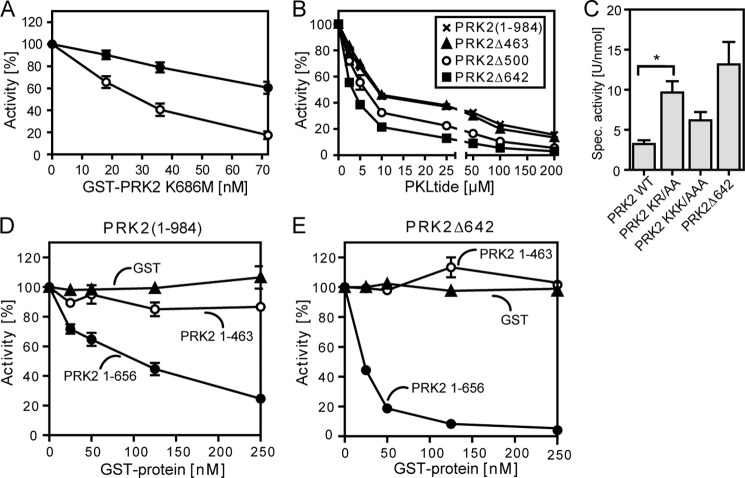
FIGURE 5.
Inhibition of the kinase activity of PRK2 by its N-terminal region. The kinase activity of wild-type and N-terminal-truncated PRK2 proteins purified from HEK 293T cells was measured in vitro using the polypeptide KKCrosstide as a substrate. A, effect of the kinase-dead mutant GST-FLAG-PRK2 K686M on the kinase activity of GST-PRK2(1–984) or GST-PRK2Δ642. B, inhibition of the kinase activity of GST-PRK2(1–984) (×), Δ463 (▴), Δ500 (○), and Δ642 (■) by a peptide comprising amino acids 487–501 (PKLQRQKKIFSKQQG; PKLtide). C, effect of mutation of basic residues in the PKL region on the kinase activity of PRK2. Basic residues at positions 488 and 491 (PRK2 KR/AA) or 494, 495, and 498 (PRK2 KKK/AAA) were mutated to alanine; *, p < 0.05 (n = 7). D and E, effect of the isolated N-terminal region with (GST-PRK2(1–656)) or without (GST-PRK2(1–463)) the PKL linker on the kinase activity of GST-FLAG-PRK2(1–984) (D) and GST-PRK2Δ642 (E) (tested at 5 nm). Purified GST was used as negative control.