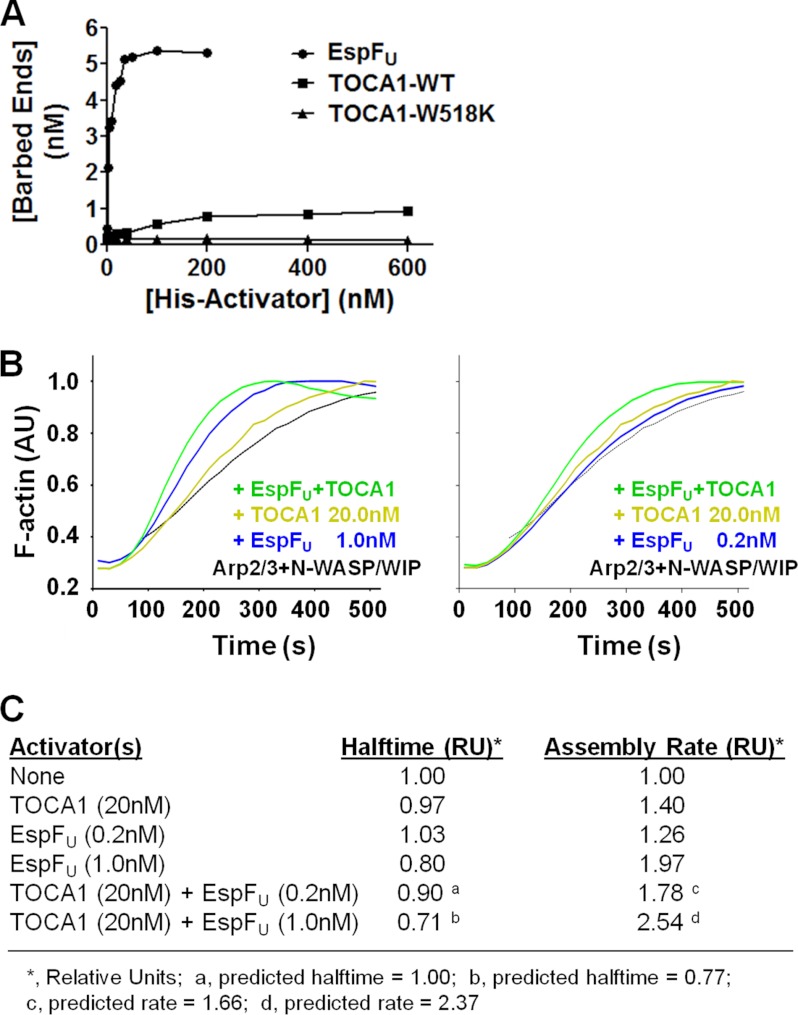
FIGURE 8.
TOCA1 and EspFU activate N-WASP/WIP additively in vitro. A, actin (2 μm) was polymerized using 20 nm Arp2/3 complex, 20 nm N-WASP/WIP complex, and the indicated concentrations of His-tagged EspFU(R1–6), TOCA1 wild type, or the W518K mutant. Barbed end concentrations were calculated when F-actin reached its half-maximal value. B, actin (2 μm) was polymerized using 20 nm Arp2/3 complex, 20 nm N-WASP/WIP complex, and the indicated concentrations of TOCA1 and EspFU(R1–6). Pyrene-actin fluorescence was measured in arbitrary units (AU) over time. C, actin (2 μm) was polymerized as in part B, and the time to reach half of the maximal F-actin concentration (Halftime) and slope of assembly curves at 60% polymer (Assembly Rate) was measured and normalized to controls containing only actin, Arp2/3 complex, and N-WASP/WIP complex. The predicted Halftimes and Assembly Rates were calculated based on the premise that the effects of TOCA1 and EspFU on actin polymerization would be additive.