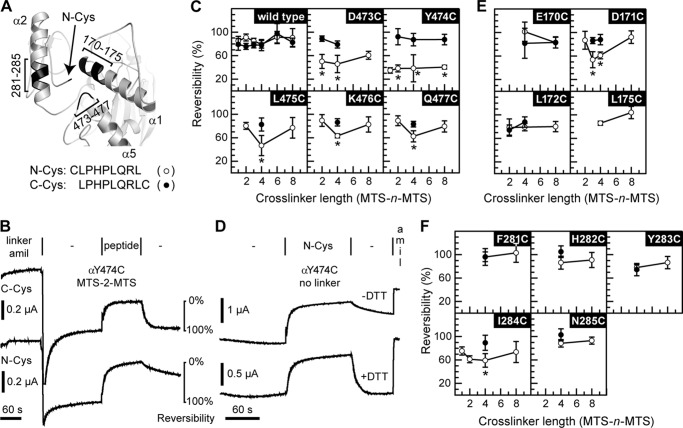
FIGURE 1.
Selected sites in the finger and thumb domains cross-link to N-Cys. A, model of ENaC α subunit finger thumb-domain interface. Position of Cys on the N terminus of the bound inhibitory peptide is indicated by an arrow. Areas predicted to be close to the N-terminal Cys are highlighted (black) and labeled. B, representative example of experiments performed in c, e, and f. Oocytes were injected with 1 ng of mutant or wild type α subunit, and 1 ng each of wild type β and γ subunits. Currents were measured by two-electrode voltage clamp at −60 mV. Channels were labeled by perfusing oocytes with 10 μm MTS-n-MTS (linker) in the presence of 20 μm amiloride (amil) for 2 min, followed by a 3-min wash (−). Channels were then inhibited by either N-Cys or C-Cys (peptide) for 2 min, followed by a 2-min wash to assess reversibility of peptide inhibition. Species tested with either N-Cys (○) or C-Cys (●) include wild type (C), mutants of the loop connecting helices α4 and α5 in the thumb (C), and mutants of helices α1 (E) and α2 (F) in the finger. Values are mean ± S.D. (n = 5–10). An asterisk indicates p < 0.01 versus the same mutant with the alternate peptide and wild type with either peptide at a given cross-linker length using ANOVA and Newman-Keuls post hoc test. D, αY474C was tested for the reversibility of N-Cys inhibition in the absence of MTS compounds. N-Cys was added to oocytes expressing the αY474C mutant for 2 min after a 2-min period establishing the base-line current. The reversibility of N-Cys inhibition after washout ± DTT was then assessed, followed by amiloride addition to determine amiloride-sensitive currents.