Figure 1.
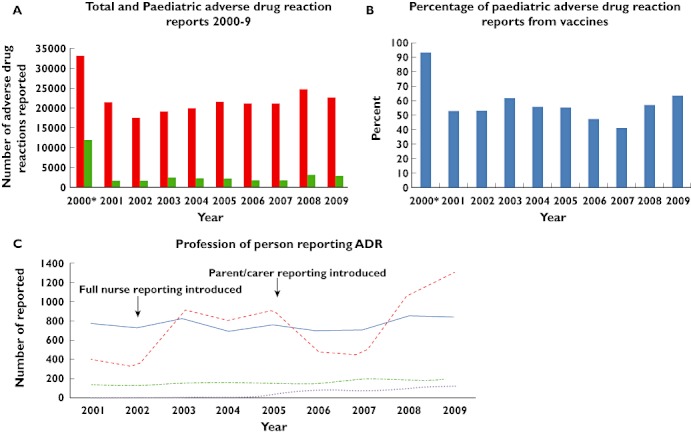
(A) Graph of the total (adult and paediatric) and paediatric (age < 17 years) adverse drug reactions (ADR) reported to the MHRA annually for the years 2000–2009 (including vaccines). (B) Graph of the percentage of paediatric ADR reports from vaccines for the years 2000–2009 (C) Graph of the number of Yellow Card reports per year grouped by profession of the reporter. Nurse reporting was initially introduced in 2000, but this was limited. Full nurse reporting was introduced in 2002, and parent and carer reporting introduced in 2005. *2000 contained a spike in reports related to Neisseria meningitidis Group C vaccine. (A) ( ) Total number; (
) Total number; ( ) Total number < 17 years; (C) (
) Total number < 17 years; (C) ( ) Doctors; (
) Doctors; ( ) Nurses; (
) Nurses; ( ) Pharmacist; (
) Pharmacist; ( ) Parent/Carer
) Parent/Carer
