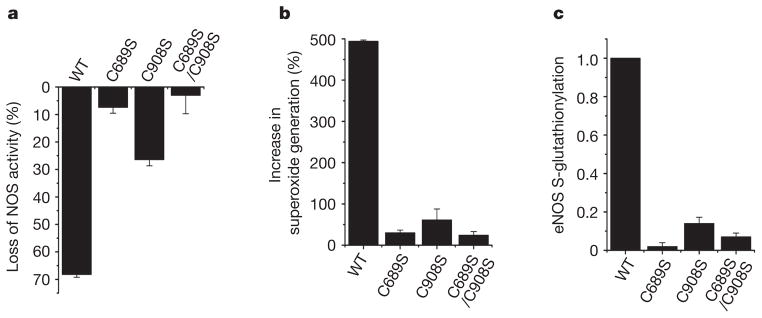
Figure 2. Cysteine mutants (C689S, C908S and C689S/C908S) of heNOS resist S-glutathionylation and secondary uncoupling.
WT heNOS and heNOS C689S, C908S and C689S/C908S mutants were treated with 2 mM GSSG. a, Percentage loss of NOS activity after treatment of heNOS with GSSG. b, Percentage increase in O2•− generation after treatment of heNOS with GSSG. c, Ratio of relative eNOS S-glutathionylation to eNOS protein. The relative intensity of eNOS S-glutathionylation/eNOS protein was normalized to the wild-type value. The Cys→Ser mutants maintained a NOS activity similar to that of the wild type (120 ± 12 n mol min−1 mg−1). Results are shown as means and s.e.m. (n =3).