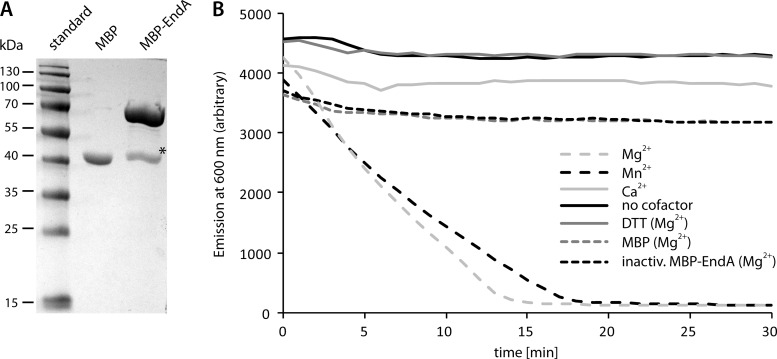
Fig 2.
Purification and in vitro activity of MPB-EndA. (A) Polyacrylamide gel electrophoresis of proteins after enrichment using amylose resin. Unfused MBP was produced and enriched in parallel (center lane). MBP-EndA migrated at a position corresponding to a molecular mass of about 70 kDa, in close agreement with the estimated mass of 73.1 kDa. A single major contaminating band (marked by an asterisk), likely representing MBP (42 kDa), was visible after enrichment. (B) Activity of highly enriched MBP-EndA on the purified vector pBluescript. The activity was determined by the loss of fluorescence of DNA-bound GelRed nucleic acid stain due to DNA degradation. Several cofactors (Mg2+, Mn2+, Ca2+) were tested, and control assays were carried out either with no cofactor, after the addition of DTT, after heat inactivation of MBP-EndA, or with MBP that was analogously produced and purified. Only the addition of Mg2+ or Mn2+ as a cofactor to EndA resulted in rapid degradation of DNA.