Fig 2.
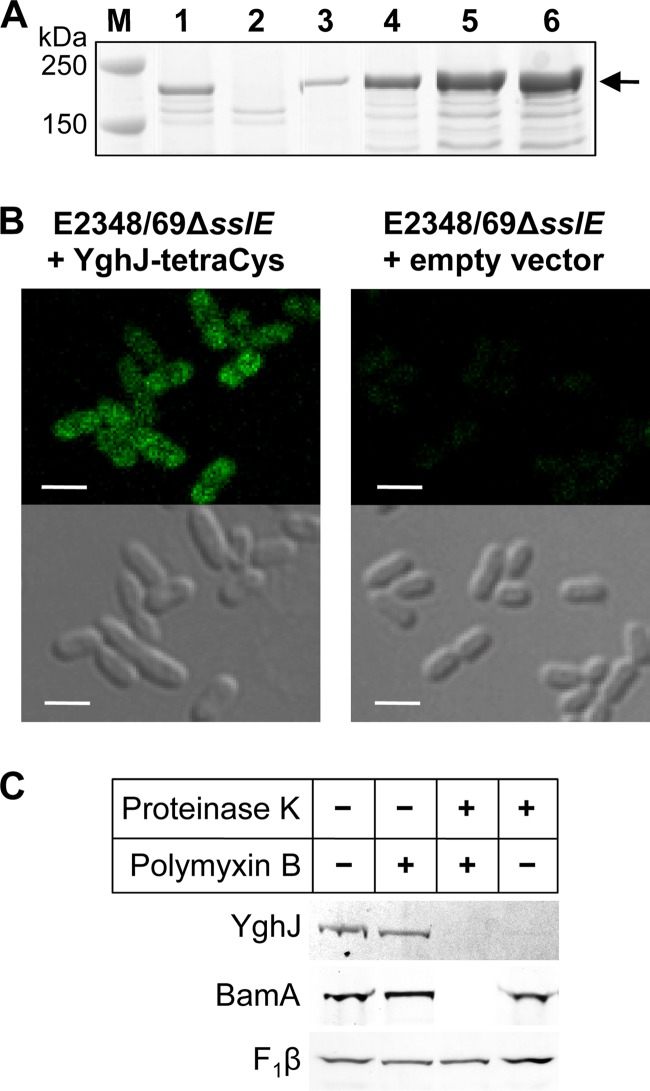
SslE-tetraCys is located on the outer surface of the outer membrane of EPEC strain E2348/69. (A) Titration of the inducer, anhydrotetracycline (ATc), to determine the amount required to induce expression of sslE-tetraCys to levels approximate to those of SslE in wild-type E2348/69. E2348/69ΔsslE(pJP169), where sslE-tetraCys was expressed from a tetA promoter, was grown in CAYE to an optical density at 600 nm (OD600) of ∼2 and then induced for 2 h with 0, 30, 35, 40, or 50 ng/ml ATc (lanes 2 to 6, respectively). Proteins were separated by SDS-PAGE using 4–12% bis-Tris NuPAGE gels (Invitrogen) and stained with Coomassie brilliant blue R-250. (B) Fluorescent and phase-contrast images of E2348/69ΔsslE carrying plasmid pJP169 (pJP168::sslE-tetraCys) or the empty vector, pJP168, which was grown in CAYE to an OD600 of ∼2 and then induced for 2 h with 35 ng/ml anhydrotetracycline. Bacteria were visualized by differential interference phase contrast on a Zeiss LSM700 inverted confocal microscope, and Lumio Green was excited using a 488-nm diode laser line. Bar, 2 μm. (C) E2348/69ΔsslE(pJP169) cells were grown as described above and incubated without (−) or with (+) polymyxin B and proteinase K, as indicated. Proteins, separated by SDS-PAGE using 4-12% bis-Tris NuPAGE gels (Invitrogen), were analyzed by Lumio Green detection for SslE and by immunoblotting for the controls, BamA (inner surface, outer membrane protein) and the cytoplasmic membrane protein FoF1 ATP synthase β-subunit (F1β). The latter served as a control for sample loading, as it is protected from proteinase K by the inner membrane.