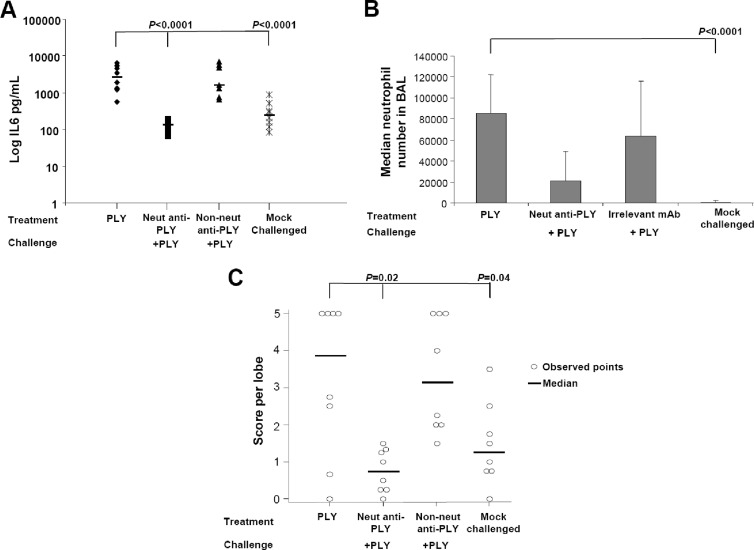
Fig 5.
Monoclonal anti-PLY neutralizing antibodies reduced in vivo PLY-mediated inflammation and toxicity. (A) Serum IL-6 levels in mice challenged i.n. with untreated PLY or PLY preincubated with either a neutralizing MAb (20 μg) or a pool of nonneutralizing anti-PLY MAbs (20 μg each). As a negative control, IL-6 levels were also determined in mock-challenged mice that received only PBS–15% glycerol i.n. (B) Neutrophil infiltration into the BAL fluid of mice challenged i.n. with untreated PLY or PLY pretreated with a neutralizing MAb or an irrelevant control. Mock-challenged mice received PBS–15% glycerol i.n. (C) Mouse lung damage scores after i.n. challenge with untreated PLY or PLY pretreated with neutralizing monoclonal antibodies. As a negative control, lung damage was also determined in mock-challenged mice that received only PBS–15% glycerol i.n. The P value refers to a significant difference between the PLY-challenged group and each indicated test group. Circles indicate data points for individual mice.