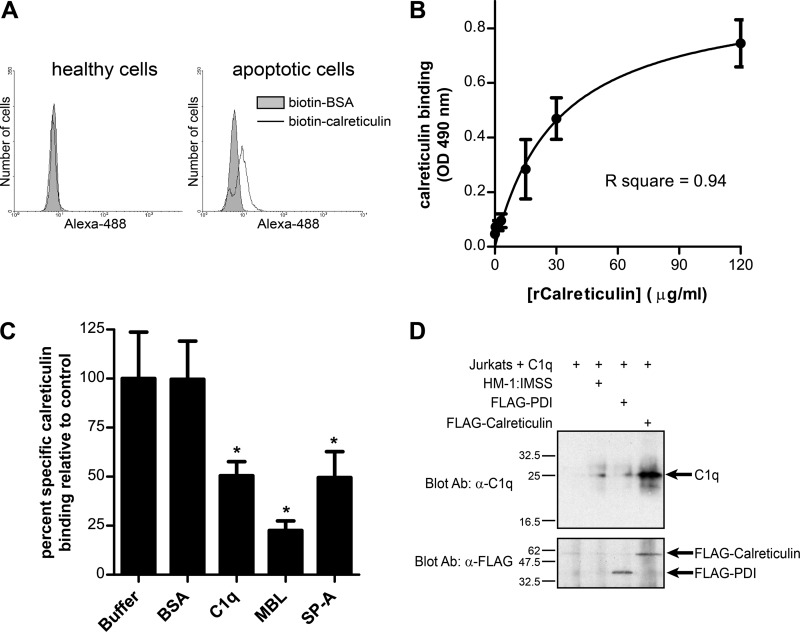
Fig 6.
E. histolytica calreticulin binds to apoptotic lymphocytes and to human C1q. (A) Calreticulin binds specifically to apoptotic lymphocytes, independent of C1q. Healthy and apoptotic Jurkat lymphocytes cultured in serum-free medium were stained for flow cytometry using biotin-calreticulin or biotin-BSA (negative control) and streptavidin-Alexa Fluor 488. Representative histograms of amebic fluorescence are shown. (B) Calreticulin binds directly to human C1q. Recombinant calreticulin (rCalreticulin) binding to immobilized C1q was measured by ELISA using biotinylated anti-calreticulin scFv and streptavidin-HRP. The graph shows a single-site specific binding curve for binding of the indicated concentration of calreticulin, generated by nonlinear regression (mean and SD; n = 3). (C) Specific inhibition of calreticulin-C1q binding by free C1q and collectins. Binding assays were performed in the presence of 3-fold molar excess BSA (negative control), C1q, MBL, and SP-A. Calreticulin binding was expressed as the percentage of specific binding measured in buffer alone (mean and SD; n = 3). *, P < 0.01 compared to buffer control by one-way ANOVA with Bonferroni correction. (D) C1q coimmunoprecipitates with FLAG-calreticulin following interaction of E. histolytica with C1q-coated apoptotic lymphocytes. Apoptotic Jurkat lymphocytes were coated with human C1q and incubated with nontransfected HM-1:IMSS trophozoites or trophozoites expressing FLAG-PDI or FLAG-calreticulin (30 min at 37°C; ameba/host cell ratio, 1.3:1) before lysis and immunoprecipitation with an anti-FLAG antibody. The top panel is an anti-C1q Western blot showing coprecipitated C1q. The bottom panel is the same membrane stripped and reprobed with the anti-FLAG monoclonal antibody. The precipitants from an equal number of amebae were run in each lane.