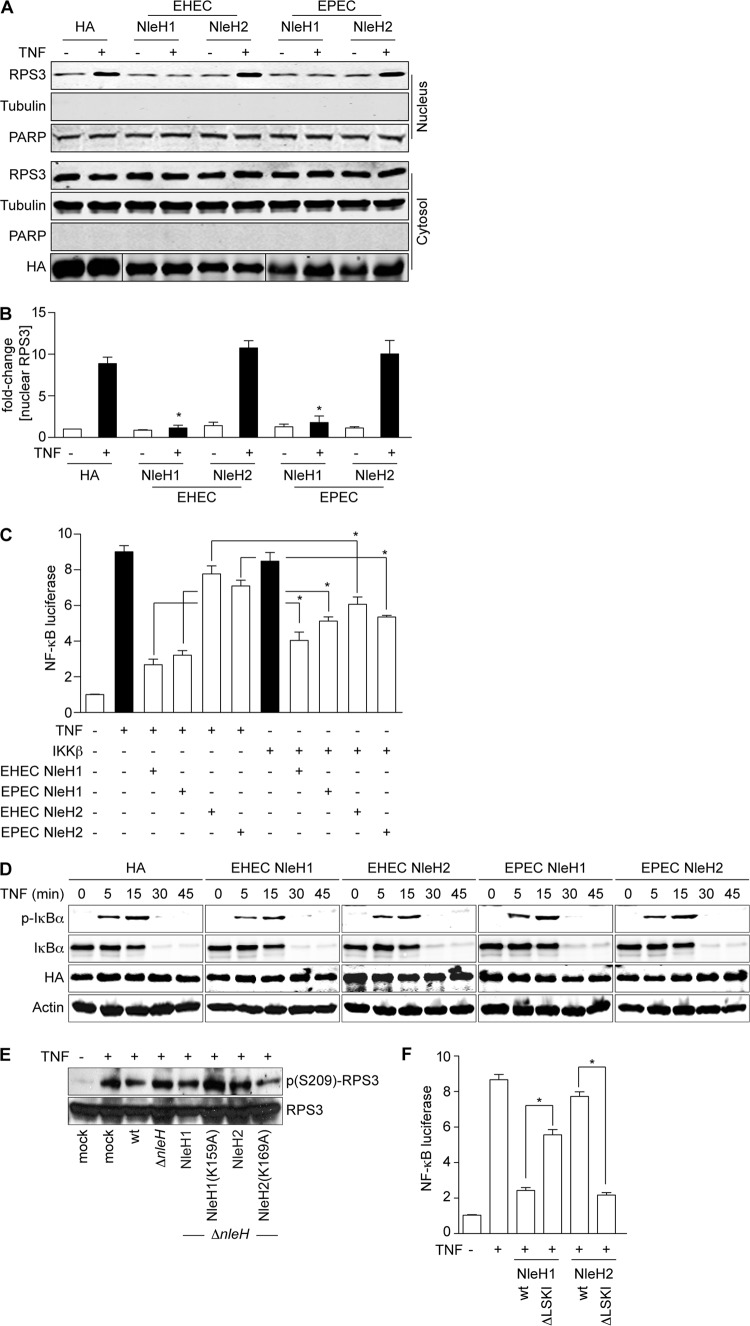
Fig 1.
EHEC and EPEC NleH proteins have similar functions. (A) RPS3 nuclear translocation. Immunoblotting of cytoplasmic and nuclear fractions of 293T cells transfected with EHEC or EPEC NleH1, NleH2, or an HA-epitope control, with (+) or without (−) TNF (20 ng/ml; 30 min). Blots were probed with RPS3, HA, tubulin, and PARP monoclonal antibodies. (B) Nuclear RPS3. Quantification (n = 4) of the fold increase in nuclear RPS3 after TNF treatment. RPS3 signal intensity was normalized to nuclear PARP. Asterisks indicate significant differences compared with HA transfection. (C) NF-κB activity. Relative NF-κB activity determined using luciferase reporter assays in 293T cells transfected with the indicated NleH plasmids (n = 3). Cells were stimulated with TNF (20 ng/ml; 30 min) or cotransfected with IKKβ, as indicated. Cells were also transfected with a firefly luciferase construct driven by a consensus κB site and with a renilla luciferase plasmid. Asterisks indicate significantly different pairwise comparisons between TNF and IKKβ stimulations. (D) Neither NleH1 nor NleH2 alters IκBα dynamics. Immunoblot analysis of total and phosphorylated IκBα after TNF (20 ng/ml; 30 min) treatment in the presence of indicated NleH constructs. (E) NleH2 does not inhibit RPS3 phosphorylation. Immunoblotting of HeLa cell lysates after a 3-h infection with C. rodentium strains complemented with the indicated EHEC NleH expression plasmids. Cells were treated with TNF (20 ng/ml; 30 min) prior to harvest. (F) PDZ-binding motif of NleH regulates its function in innate immunity. Relative NF-κB activity determined using luciferase reporter assays in 293T cells transfected with the indicated NleH plasmids. Experiments were conducted as described for panel C. Asterisks indicate significantly different pairwise comparisons between wild-type and ΔLSKI constructs.