Fig 1.
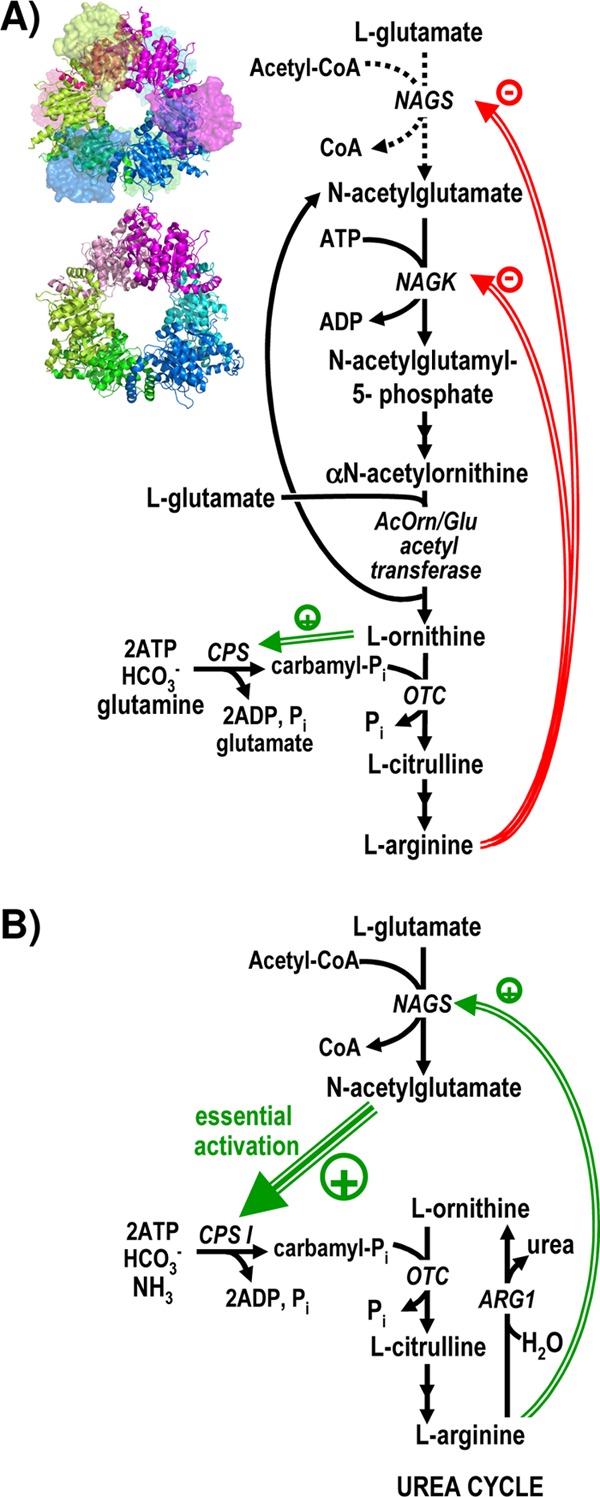
Roles of N-acetyl-l-glutamate synthase (NAGS) and of its homologous enzyme N-acetyl-l-glutamate kinase (NAGK) in arginine biosynthesis. Two arrows in succession indicate the existence of two steps that are not detailed. Double and triple green arrows and green-encircled plus signs denote activation, whereas double red arrows and red-encircled minus signs denote inhibition. OTC, ornithine transcarbamylase. ARG1, arginase 1. (A) Schematic representation of the arginine biosynthetic pathway of P. aeruginosa and of many other bacteria and plants. The dotted arrows for the NAGS reaction indicate an anaplerotic role of NAGS in those organisms, like P. aeruginosa, in which the N-acetyl group is recycled by transacetylation from acetylornithine to glutamate (10). However, some organisms, like E. coli, deacetylate acetylornithine hydrolytically, and in these cases, NAGS makes one NAG molecule per arginine molecule synthesized (10). The structures of the NAGS from N. gonorrhoeae (34) (PDB accession number 2R8V) and of the NAGK from P. aeruginosa (28) (PDB accession number 2BUF) are shown next to the steps catalyzed by them to grossly illustrate their structural similarity. They are viewed along their 3-fold axes, with each dimer colored differently and with both subunits of each dimer in different hues. NAGK and the AAK domain of NAGS are shown in a cartoon representation. In NAGS, to avoid occluding the view of the AAK domains, the GNAT domains are shown in a surface semitransparent representation, and those in the background are fainter. (B) Arginine and urea biosynthesis in urea-making terrestrial animals such as humans. Animals do not make ornithine through N-acetylated intermediates, and they lack NAGK and other enzymes of the route except NAGS (2). The large green triple arrow stresses the essentiality of the activation of carbamoyl phosphate synthetase (CPS I) by NAG.
