Fig 5.
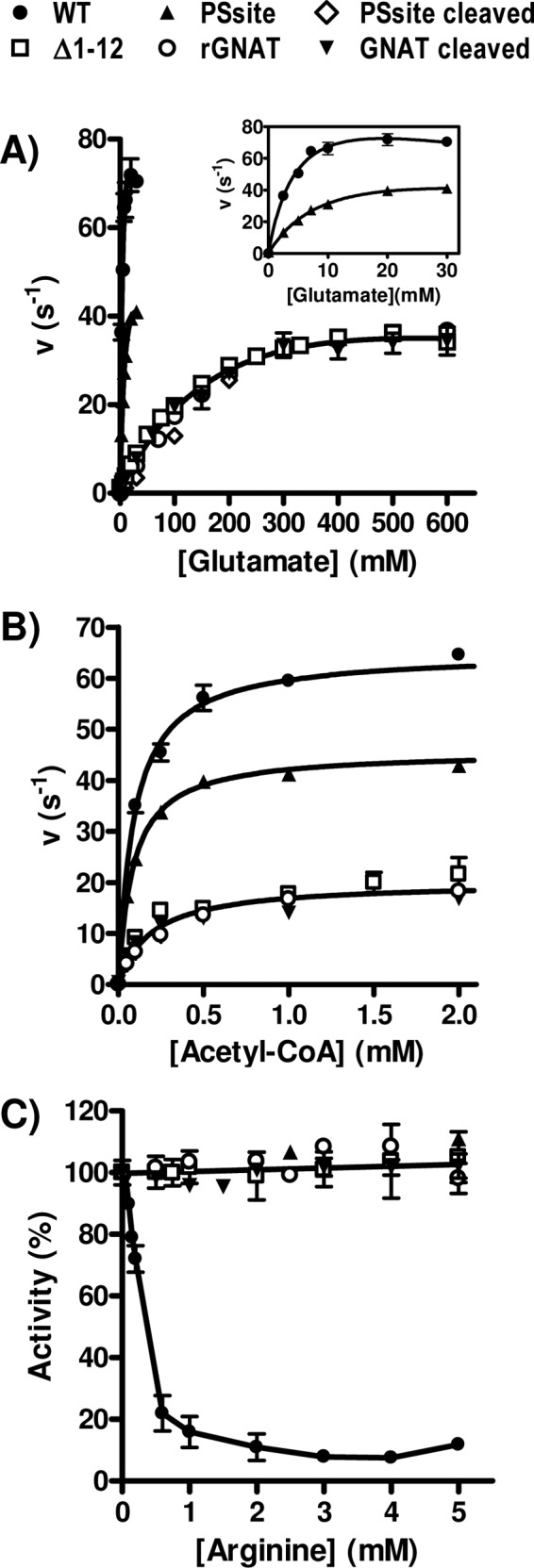
Dependency of NAGS activity on the concentration of both substrates (A and B) and of arginine (C) for the WT, the PSsite form (either noncleaved or PreScission protease cleaved), and the Δ1–12 form of PaNAGS and of the GNAT domain either isolated after cleavage (GNAT cleaved) or produced recombinantly (rGNAT). When the concentration of acetyl-CoA was varied, the concentration of glutamate was fixed at 100 mM, except for the WT and the noncleaved PSsite forms, with which it was kept at 30 mM. When the concentration of glutamate was varied, the concentration of acetyl-CoA was fixed at 4 mM. To allow a meaningful comparison of the activities of various enzyme forms having different masses, velocities are given as turnover numbers per polypeptide chain (units are s−1). A similar concentration dependency of the PSsite-cleaved form, the Δ1–12 form, and the isolated GNAT domains is evident for each substrate. (A and B) Therefore, single curves were fitted for the results for all these forms for glutamate (A) and for acetyl-CoA (B). The curve for glutamate corresponds to hyperbolic kinetics with substrate inhibition and with apparent values of KmGlu, KIGlu, and kcat at infinite glutamate of 240 ± 45 mM, 1,254 ± 480 mM, and 66 ± 8 s−1, respectively. The curve for acetyl-CoA (B) is a hyperbola, with apparent values of Kmacetyl-CoA and kcat at infinite acetyl-CoA of 190 ± 30 μM and 20.0 ± 0.7 s−1, respectively. The KmGlu, KIGlu, and Kmacetyl-CoA values for the WT and for the noncleaved PSsite forms of the enzyme are those shown in Table 1, and the apparent kcat values are 111 ± 16 s−1 for the WT and 69 ± 7 s−1 for the noncleaved PSsite form (see inset in panel A) for infinite glutamate and 65 ± 1 s−1 for the WT and 46 ± 1 s−1 for the PSsite form for infinite acetyl-CoA (see panel B). (C) Influence of arginine concentration on enzyme activity. Results are expressed as a percentage of the activity of the same enzyme form in the absence of arginine. A single line corresponding to no inhibition has been fitted to the results for all forms except the wild-type enzyme. Substrate concentrations in these assay mixtures were 4 mM acetyl-CoA and either 30 mM glutamate for the WT and noncleaved PSsite forms or 100 mM glutamate for all other forms.
