Fig 1.
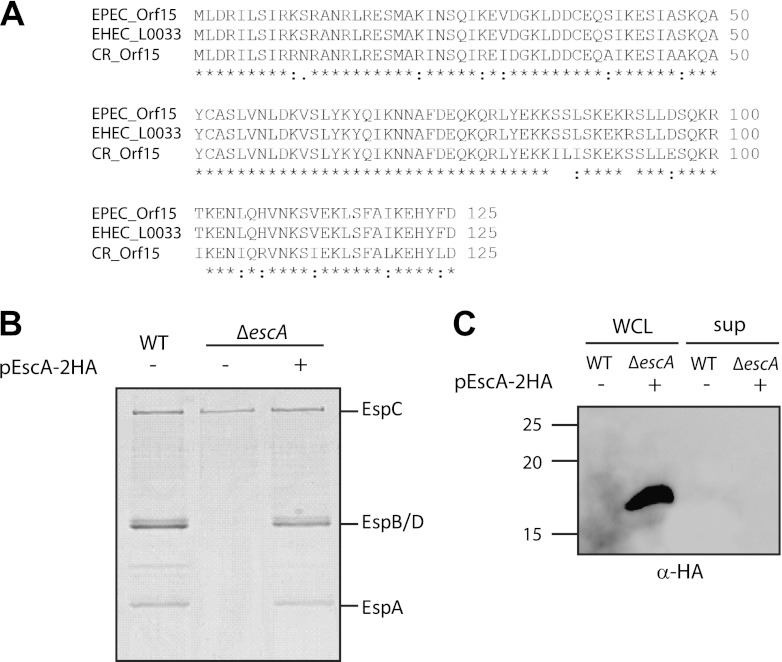
EscA is essential for T3S. (A) BLAST (http://blast.ncbi.nlm.nih.gov) and multiple alignment (using CLUSTAL W) of Orf15 from EPEC with T3S proteins of the related A/E pathogens, enterohemorrhagic (EHEC) E. coli (O157:H7) and C. rodentium (ICC1680). Accession numbers are as follows: EPEC Orf15, gb|AAC38384.1; EHEC (E. coli O157:H7) L0033, NP_290267.1; C. rodentium [CB] Orf15, gb|AF311901.1. An asterisk indicates positions which have a single, fully conserved residue; a colon indicates conservation between groups of strongly similar properties; and a period indicates conservation between groups of weakly similar properties. (B) Protein secretion profiles of WT EPEC, a ΔescA strain, and a ΔescA strain complemented with escA in trans. Secreted proteins were concentrated from supernatants of bacterial cultures grown in DMEM and analyzed by Coomassie staining of an SDS–12% PAGE gel. The locations of the translocators EspA, EspB, and EspD are indicated at the right of the gel. Also indicated is the location of EPEC EspC, which is not secreted via the LEE-encoded T3SS. (C) Whole-cell lysates (WCL) and secreted proteins (sup) of an EPEC WT and a ΔescA strain complemented with escA grown under T3S-inducing conditions were examined for EscA secretion. Samples were analyzed by SDS–16% PAGE and immunoblotting using an anti-HA antibody. Molecular size markers (kDa) are indicated at the left of the gel.