Abstract
Cerebral nocardiosis is a severe infection that carries the highest mortality rate among all bacterial cerebral abscesses. We report on a case in an immunocompromised patient which was successfully treated with unexpectedly low doses of linezolid. Therapeutic drug monitoring was very helpful in highlighting issues of poor compliance and of drug-drug interactions.
TEXT
Nocardiosis is a rare but severe infectious disease which usually affects patients with deficient cell-mediated immunity (1). Although the primary site of infection is usually the lung, general dissemination with secondary involvement of the central nervous system (CNS) may sometimes occur (11), with abscess formation as a sign of microorganism tissue indwelling. The first-line treatment of CNS nocardiosis is trimethoprim-sulfamethoxazole, even for up to 1 year, but antibiotic discontinuation due to a high incidence of adverse drug reactions or to breakthrough resistance to sulfonamides is frequently needed (1). In these cases, neurosurgery to save the patient's life is often unavoidable, sometimes with relevant sequelae (11).
Linezolid, an oxazolidinone with time-dependent activity, has proved effective for the treatment of Nocardia infections, even in the presence of cerebral dissemination (9). Although no dosage adjustment in the presence of renal or hepatic failure is required, in the last few years, it has been reported that some drug-drug interactions may alter its pharmacokinetic behavior, so that therapeutic drug monitoring (TDM) for optimization of drug exposure has been advocated (13). This may be especially useful in prolonged antimicrobial courses when drug-related adverse events like anemia, thrombocytopenia, and lactic acidosis may raise concerns (16).
Here we describe a case of cerebral nocardiosis successfully treated for a long time with unexpectedly low doses of linezolid in a patient receiving complex polytherapy.
A 67-year-old woman was diagnosed with cavitary pneumonia while undergoing treatment with cyclosporine plus prednisone because of cold agglutinin haemolytic anemia. After 3 weeks of wide-spectrum antibiotic therapy (with piperacillin-tazobactam plus vancomycin) with no radiological or clinical response and because of negative bronchoalveolar lavage fluid cultures, Aspergillus pneumonia was suspected and treatment with voriconazole was started. At that time, a diagnostic workup confirmed that no other body site was involved in any infective process.
Approximately 1 month later, while on antimicrobial treatment, she was admitted to the emergency department because of aphasia and confusion, and while being examined, she experienced an epileptic seizure which resolved spontaneously. Brain computed tomography imaging revealed a single left posterior temporo-parietal plurinodular abscess with a perilesional edematous area, which was subsequently confirmed by nuclear magnetic resonance (NMR) imaging (Fig. 1A). A stereotactic brain biopsy of the aforementioned lesion allowed the identification of modified acid-fast variable branching filamentous bacteria with a morphology consistent with Nocardia species. In light of this, given the trends toward increasing resistance of Nocardia to sulfonamides in Northern Italy (5), antimicrobial therapy was switched to linezolid at 600 mg every 12 h (q12h) (day 0), also taking into account the excellent CNS penetration of this drug (10). After starting therapy, the neurological and general status of the patient improved, and 2 weeks later, she was discharged from the hospital still undergoing treatment with linezolid and followed up with a clinical evaluation every 2 weeks.
Fig 1.
Cerebral NMR imaging at baseline (A) and 1 year after the end of treatment with linezolid (B).
After 1 month, assessments of hematological parameters revealed a progressive decline from the baseline of red blood cell (RBC) count (from 3.54 × 106/mm3 to 2.3 × 106/mm3), of hemoglobin level (from 11.8 g/dl to 7.82 g/dl), and of platelet (PLT) count (from 349 × 103/mm3 to 221 × 103/mm3).
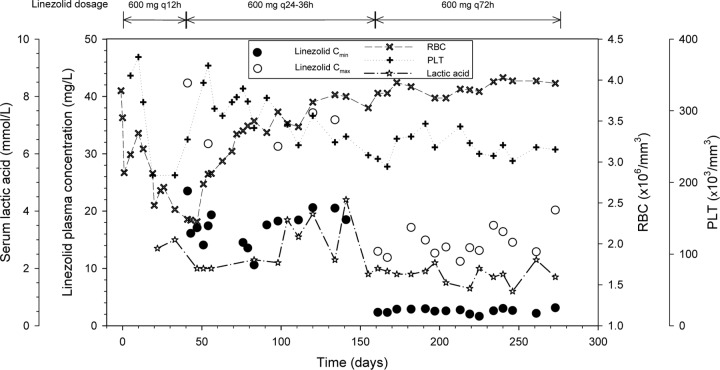
Since drug-related toxicity was suspected, TDM was performed on day 41, and very high trough (Cmin) and peak (Cmax) concentrations of 23.49 and 42.33 mg/liter, respectively, were assessed. Serum linezolid concentrations were measured using high-performance liquid chromatography by the UV detection technique as previously described (13). A dosage reduction to 600 mg q48h was promptly recommended with the intent of maintaining the Cmin between 2 and 10 mg/liter. Temporal trends of plasma linezolid concentrations, RBC and PLT counts, and lactic acid levels are depicted in Fig. 2.
Fig 2.
Temporal trends of plasma linezolid Cmin and Cmax, serum lactic acid levels, and RBC and PLT counts.
Surprisingly, repeated TDM revealed that at subsequent points the linezolid Cmin persisted over time unexpectedly far above the desired range (mean Cmin of 16.9 ± 2.8 mg/liter). At that time, the patient was undergoing complex polytherapy (carvedilol, barnidipine, doxazosin, cyclosporine, prednisone, oxcarbazepine, and nortriptyline-fluphenazine) due to multiple chronic comorbidities. Careful counseling of the patient and her relatives was undertaken for complete reevaluation of the dosage schedule of each individual drug. It emerged that the patient was autonomously taking omeprazole and that the true doses of linezolid were larger than those prescribed (varying between 600 mg q24h and 600 mg q36h), as a consequence of poor compliance due to the significant pill burden.
After stopping omeprazole and reducing the linezolid dose to 600 mg q72h while maintaining the other coadministered drugs unmodified, the Cmin definitively normalized at day 161 and persisted within the desired range (mean Cmin of 2.56 ± 0.4 mg/liter) until the end of therapy (day 273), with progressive recovery from hematological toxicity and from hyperlactacidemia. A complete clinical response was obtained, and a cure was confirmed by NMR imaging at the 1-year follow-up (Fig. 1B).
Cerebral nocardiosis is a very severe infection that carries the highest mortality rate among all bacterial cerebral abscesses, especially in immunocompromised patients (11). From the available literature (6, 9, 11), it appears that medical treatment of this condition is particularly challenging in terms of both drug selection for effective penetration of the cerebral parenchyma and the tolerability of lengthy antimicrobial courses. Although sulfonamides have been the drugs of choice for several years, given the increasing number of reports of resistance (5, 9) and the relatively high incidence of adverse events occurring during sulfonamide therapy (1), linezolid has recently gained relevance (6, 9, 11), mainly as rescue therapy, in light of its effective in vitro activity against Nocardia spp. (9) and of its excellent CNS penetration (10). Linezolid is one of the few antimicrobials to be active in vitro against all clinically significant species of the genus Nocardia (3). In a study assessing the susceptibility of 140 Nocardia isolates to linezolid (4), the MIC50 and MIC90 for all species other than Nocardia farcinica were 2 and 4 mg/liter, respectively, whereas those for N. farcinica were both 4 mg/liter.
Among 11 cases of nocardiosis treated with linezolid (6), a clinical cure (9/11) or marked improvement (2/11) was always reported, but hematological toxicity was documented in up to 45% of the patients. Indeed, mild-to-moderate thrombocytopenia and/or anemia due to transient reversible bone marrow suppression may be expected in prolonged treatment with linezolid (16). However, it has been shown that these adverse events, as well as hyperlactacidemia due to interference with mitochondrial protein synthesis, may occur more frequently in the presence of drug overexposure (14, 15).
In our patient, the appearance of both hematological toxicity and hyperlactacidemia after 40 days of standard treatment with 600 mg of linezolid q12h was clearly caused by significant overexposure.
As far as the possible causes of this are concerned, our attention focused mainly on pharmacokinetic drug-drug interaction. It has been recently suggested that linezolid may be a substrate of P-glycoprotein (P-gp) and that significant overexposure may occur in the presence of some P-gp inhibitors, namely, omeprazole, amiodarone, and amlodipine (13). Interestingly, three of the drugs of the complex polytherapy of our patient, namely, omeprazole, barnidipine, and carvedilol, are potent P-gp inhibitors (2, 8, 12). Of note, the half-maximal inhibitory concentration (IC50) of omeprazole against P-gp-mediated transport was found to be 17.7 μM, which is higher than its expected plasma concentrations at therapeutic doses, but not if considering poor metabolizers of CYP2C19 (12). This suggests that the linezolid-omeprazole interaction could be particularly relevant among poor metabolizers of CYP2C19. However, it has also been found that for barnidipine and carvedilol the IC50s for inhibition of P-gp-mediated transport were even lower (8.6 μM for barnidipine [7] and 0.16 μM for carvedilol [2]) and in the range of their respective plasma concentrations. This suggests that linezolid overexposure could be particularly relevant among patients who receive cotreatment with all of these different P-gp inhibitors. Of note, in our patient, the final linezolid dose was 6-fold lower than the standard one, and this suggest that the concomitant administration of omeprazole, barnidipine, and carvedilol may have had a cumulative effect in reducing linezolid clearance.
We wondered about the persistence of drug overexposure in spite of the conspicuous dosage reduction suggested. Indeed, we recognize that the careful counseling done, which revealed that the autonomous assumption of omeprazole and poor patient compliance were the leading causes of this, was unfortunately carried out only after 3 more months of therapy. This fact clearly highlights the damaging effects that self-medication may have on elderly patients undergoing complex polytherapy. Additionally, it suggests that giving the correct information about the dosage schedules of the various drugs not only to the patient but also to the patient's relatives may be very helpful in avoiding drug-related inconveniences.
The progressive disappearance of drug-related toxicity, coupled with the achievement of a complete clinical cure in spite of apparently low doses of linezolid, clearly suggests that TDM-guided dose reduction played a major role in the safety of long-term treatment with linezolid in this case.
Besides, maintenance over time of a Cmin of >2 mg/liter, which is a value above the MIC50 of linezolid against most of the clinically significant Nocardia species (4, 9), allowed a clinical cure. This is in agreement with linezolid being a time-dependent agent whose antibacterial activity may be maximized when concentrations are persistently maintained above the MIC for the pathogen for the entire dosing interval (13).
It should not be overlooked that although linezolid appears to be an effective alternative for the treatment of nocardiosis, this indication remains exceptional. Prolonged linezolid use for more than 28 days could increase the likelihood of selection for antimicrobial resistance, especially in the presence of underexposure, and may cause hematological toxicity, especially in the presence of overexposure. However, considering that all of the adverse events of long-term linezolid therapy may be drug exposure related, TDM may represent the absolute solution of these concerns.
In conclusion, our experience suggests that in patients requiring prolonged treatment with linezolid and receiving complex polytherapy TDM coupled with careful ambulatory counseling may be of paramount relevance either in ensuring efficacy while avoiding the risk of premature discontinuation of therapy due to adverse events or in highlighting issues of poor compliance.
ACKNOWLEDGMENTS
No financial support was received for this study.
Federico Pea has been on the speakers' bureau of Pfizer. Mario Furlanut has received grant support from Pfizer. Pierluigi Viale has been a consultant to, has been on the speakers' bureau of, and has received grant support from Pfizer. Francesco Cristini has been on the speakers' bureau of Pfizer. The rest of us have no potential conflict of interest to report.
Footnotes
Published ahead of print 27 February 2012
REFERENCES
- 1. Ambrosioni J, Lew D, Garbino J. 2010. Nocardiosis: updated clinical review and experience at a tertiary center. Infection 38:89–97 [DOI] [PubMed] [Google Scholar]
- 2. Bachmakov I, Werner U, Endress B, Auge D, Fromm MF. 2006. Characterization of beta-adrenoceptor antagonists as substrates and inhibitors of the drug transporter P-glycoprotein. Fundam. Clin. Pharmacol. 20:273–282 [DOI] [PubMed] [Google Scholar]
- 3. Brown-Elliott BA, Brown JM, Conville PS, Wallace RJ., Jr 2006. Clinical and laboratory features of the Nocardia spp. based on current molecular taxonomy. Clin. Microbiol. Rev. 19:259–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brown-Elliott BA, et al. 2001. In vitro activities of linezolid against multiple Nocardia species. Antimicrob. Agents Chemother. 45:1295–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Farina C, Boiron P, Goglio A, Provost F. 1995. Human nocardiosis in northern Italy from 1982 to 1992. Northern Italy Collaborative Group on Nocardiosis. Scand. J. Infect. Dis. 27:23–27 [DOI] [PubMed] [Google Scholar]
- 6. Jodlowski TZ, Melnychuk I, Conry J. 2007. Linezolid for the treatment of Nocardia spp. infections. Ann. Pharmacother. 41:1694–1699 [DOI] [PubMed] [Google Scholar]
- 7. Katoh M, Nakajima M, Yamazaki H, Yokoi T. 2001. Inhibitory effects of CYP3A4 substrates and their metabolites on P-glycoprotein-mediated transport. Eur. J. Pharm. Sci. 12:505–513 [DOI] [PubMed] [Google Scholar]
- 8. Katoh M, Nakajima M, Yamazaki H, Yokoi T. 2000. Inhibitory potencies of 1,4-dihydropyridine calcium antagonists to P-glycoprotein-mediated transport: comparison with the effects on CYP3A4. Pharm. Res. 17:1189–1197 [DOI] [PubMed] [Google Scholar]
- 9. Moylett EH, et al. 2003. Clinical experience with linezolid for the treatment of nocardia infection. Clin. Infect. Dis. 36:313–318 [DOI] [PubMed] [Google Scholar]
- 10. Myrianthefs P, et al. 2006. Serum and cerebrospinal fluid concentrations of linezolid in neurosurgical patients. Antimicrob. Agents Chemother. 50:3971–3976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ntziora F, Falagas ME. 2007. Linezolid for the treatment of patients with central nervous system infection. Ann. Pharmacother. 41:296–308 [DOI] [PubMed] [Google Scholar]
- 12. Pauli-Magnus C, Rekersbrink S, Klotz U, Fromm MF. 2001. Interaction of omeprazole, lansoprazole and pantoprazole with P-glycoprotein. Naunyn Schmiedebergs Arch. Pharmacol. 364:551–557 [DOI] [PubMed] [Google Scholar]
- 13. Pea F, et al. 2010. Therapeutic drug monitoring of linezolid: a retrospective monocentric analysis. Antimicrob. Agents Chemother. 54:4605–4610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pea F, et al. 2006. Hyperlactacidemia potentially due to linezolid overexposure in a liver transplant recipient. Clin. Infect. Dis. 42:434–435 [DOI] [PubMed] [Google Scholar]
- 15. Tsuji Y, et al. 2011. Thrombocytopenia and anemia caused by a persistent high linezolid concentration in patients with renal dysfunction. J. Infect. Chemother. 17:70–75 [DOI] [PubMed] [Google Scholar]
- 16. Vinh DC, Rubinstein E. 2009. Linezolid: a review of safety and tolerability. J. Infect. 59(Suppl. 1):S59–S74 [DOI] [PubMed] [Google Scholar]